Properties of GABA receptors
GABA (g-aminobutyric acid) is the main inhibitory neurotransmitter in the central nervous system. The inhibitory action of GABA is mediated by the receptors present on the cell membrane, and results in a reduction of neuronal excitablity. At least three types of GABA receptors have been characterized. Table 1 summarizes some of the general properties of these three types of GABA receptors. GABAA receptors are ligand-gated chloride channels. They mediate fast inhibition and have a wide distribution throughout the central nervous system. GABAcreceptors have a divese molecular composition. At least 16 subunits in 6 groups have been identified. Pharmacologically, these receptors are antagonized by bicuculline. GABAA receptors are also the targets of many therapeutic compounds (such as general anaesthetics, sedative drugs, and alcohols). These compounds allosterically modulate GABAA receptor channel activities. GABAB receptors belong to the G-protein coupled receptor superfamily. The inhibition of GABAB receptors is mediated by indirect gating of either potassium or calcium channels. GABAB receptors are activated by baclofen, and antagonized by phaclofen and saclofen. The subunits of GABAB receptors have recently been cloned. GABAc receptors are the newly identified member of the GABA receptor family. They are also linked to chloride channels, with distinct physiological and pharmacological properties. In contrast to the fast and transient responses elicited from GABAA receptors, GABAc receptors mediate slow and sustained responses. Pharmacologically, GABAc receptors are bicuculline- and baclofen-insensitive, and are not modulated by many GABAA receptor modulators (such as benzodiazepines and barbiturates). GABA r subunits are thought to participate in forming GABAc receptors on the neuronal membrane, but the exact molecular composition of these receptors is yet to be determined. GABAcreceptors are expressed in many brain regions, with prominent distributions on retinal neurons, suggesting these receptors play important roles in retinal signal processing.
Table 1. Characteristics of GABA Receptors
| GABAA Receptor | GABAB Receptor | GABAC Receptor | |
| Category | Ligand-gated Channel | G-protein coupled receptor | Ligand-gated Channel |
| Subunits | a, b, g, d, e, p | GBR1, GBR2 | r |
| Agonists | Muscimol, THIP | Baclofen | |
| Antagonists | Bicuculline, Picrotoxin | Phaclofen | TPMPA, Picrotoxin |
| Desensitization | Yes | No | No |
| Modulator | Benzodiazepines Barbiturates | Zinc |
GABAc responses on retinal neurons
The term “GABAc receptor” was first used by Johnston to describe a novel GABA binding site on neuronal membranes (Johnston, 1986, 1996). Although recent studies indicate a wide distribution of GABAc receptors in many parts of the central nervous system (Sivilotti and Nistri, 1991; Albrecht et al., 1997; Boue-Grabot et al., 1998; Wegelius et al., 1998; Enz and Cutting, 1999), these receptors are most prominently expressed in the vertebrate retina. In the fish white perch retina, rod-driven (H4) horizontal cells were the first retinal neurons where GABAc receptors were characterized (Qian and Dowling, 1993). Subsquently, GABAc-receptor mediated responses have been detected in many types of retinal neurons, including bipolar cells (Feigenspan et al., 1993; Qian and Dowling, 1995; Qian et al., 1997b; Lukasiewicz et al., 1994; Lukaisiewicz and Wong, 1997; Pan and Lipton, 1995; Nelson et al., 1999), cone-driven horizontal cells in catfish (Dong et al, 1994; Kaneda et al., 1997), cone photoreceptors (Picaud et al, 1998), and ganglion cells (Zhang and Slaughter, 1995). Among all these retinal neurons, the rod-driven horizontal cells of white perch are the only cells where GABA responses are mediated solely by GABAc receptors. The GABA responses elicited from other cells are usually a mixture of GABA receptors and/or GABA transporters. The unique properties of the white perch rod-driven horizontal cell provided an excellent model to characterize the physiological and pharmacological properties of GABAc receptors on retinal neurons (Qian and Dowling, 1993, 1994).
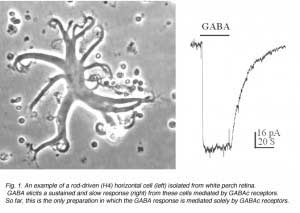
An example of a solitary rod-driven horizontal cell isolated from white perch retina is shown in Fig. 1, together with a typical GABA-elicited response from such a cell. These horizontal cells receive input from rod photoreceptors in the retina; and when isolated, they keep their typical morphology in culture. Rod-driven horizontal cells have a flat cell body with diameter of 50-100 um. There are several thick primary dendrites from which many fine processes extend. As shown in Fig. 1, GABA elicits a slow and sustained response from these cells. The GABA-induced membrane currents are mediated by chloride ions, and therefore, exhibit inhibitory actions on these neurons. The responses showed no sign of desensitization, i.e. the responses are maintained at a steady level as long as GABA is present. Furthermore, the GABA responses elicited from these horizontal cells exhibits slow kinetics, which could best be observed in the offset response. After the termination of GABA application, the membrane current returns to the baseline very slowly, with a time constant of ~15 seconds. Such slow and sustained response properties are typical of GABAc receptors.
It is interesting to note that the neurons in distal retina (i.e. photoreceptors, horizontal cells and bipolar cells) do not produce action potentials at all. They only generate slow graded responses to light stimuli. It has always been thought that retinal neurons must have special ways to process and analyze such slow signals compared with fast transient neurons of the brain. The kinetics of GABAcreceptor mediated responses are thus particularly suited for the generatation of signals in distal retinal neurons.
Pharmacology of GABAc receptors
GABAc receptors exhibit a distinct pharmacology that differs from classical GABAA or GABAB receptors. GABAc receptors were first described by Johnston for bicuculline- and baclofen-insensitive GABA binding sites on neuronal membranes (Johnston, 1986, 1996). More detailed studies indicated that GABAc receptors on retinal neurons are not sensitive to the competitive antagonists of either GABAA receptors (such as SR95531 and hydrastine), or GABAB receptors (such as phaclofen and saclofen). Since the competitive antagonists are thought to interact with the GABA binding sites on the receptors, these results indicate that a different conformation of GABA molecule is preferred for binding to the GABAc receptor. In agreement with such a notion, the specific agonists of GABAA and GABAB receptors exhibit quite different activity on GABAc receptors. They either have no effect (isonipecotic acid, baclofen), or act as partial agonists (isoguvacine, muscimol), or as antagonists (THIP, P4S, 3-APA and 3-APMPA) (Woodward et al., 1993; Qian and Dowling, 1994). I4AA, a partial agonist of GABAA receptors, behaves as a potent antagonist on GABAc responses of retinal neurons, and as a partial agonist on expressed GABAc receptors in Xenopus oocytes (Qian and Dowling, 1994; Qian et al., 1998).
GABAc receptors also differ from classical GABAA receptors in terms of their responses to various modulators. Two groups of compounds, benzodiazepine and barbiturates, are well-known to modulate GABAA activity. On the other hand, none of these compounds have any significant influence on responses mediated by GABAc receptors (Polenzani et al., 1991). For example, the GABA elicited response on white perch rod-driven horizontal cells are virtually identical in the presence or absence of either diazepam or pentobarbital (Qian and Dowling, 1993). Another class of GABAA receptor modulators, known as neuroactive steroids, have different effects on the expressed GABAc receptor in Xenopus oocytes. Whereas some of them modulate GABA responses on these receptors, others do not (Woodward et al., 1992a; Morris et al., 1999). The effect of these neuroactive steroids on GABAc receptors on neurons has yet to be determined.
Although both GABAA and GABAc receptors are linked to chloride channels, the channel properties of these two receptors are quite different. Unlike GABAA receptors, picrotoxin inhibition on GABAcreceptors in white perch horizontal cells exhibits both competitive and non-competitive mechanisms (Qian and Dowling, 1994). In mammalian retina (rat), on the other hand, GABAc receptors are insensitive to picrotoxin blockage (Feigenspan et al., 1993; Pan and Lipton, 1995). The unusual features of GABAc receptors in rat retina are attributed to a single amino acid substitution in the receptor subunit (Zhang et al., 1995). Furthermore, TBPS, like picrotoxin another chloride channel blocker on GABAA receptors, does not block the responses mediated by GABAc receptors (Qian and Dowling, 1994; Woodward et al., 1992b). In addition, GABAc receptor-gated chloride channels exhibit a very small single channel conductance (Qian and Dowling, 1995; Chang and Weiss, 1999).
GABAc receptor activities are modulated by divalent cations (Calvo et al., 1994; Kaneda et al., 1997; Dong and Werblin, 1995; Chang et al., 1995). In particular, GABAc receptor-mediated responses are inhibited by low concentrations of zinc ions. The high sensitivity of GABAc receptors to zinc inhibition is attributed to a histidine residue on the extracellular domain of the subunits (Wang et al., 1995).
Recently, a new GABAc receptor antagonist, TPMPA, has become available commercially. This compound is thought to be a specific inhibitor of the GABAc receptor (Ragozzino et al., 1996). The availability of such a drug will great facilitate further studies of this receptor.
Molecular biology of GABAc receptors
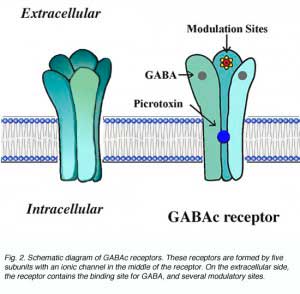
The GABAc receptor is a member of the ligand-gated channel superfamily. By analogy to the well studied nicotinic acytocholine receptors, GABAc receptors are thought to exhibit the structure shown schematically in Fig. 2. These receptors are pentomers, i.e. five subunits constitute the functional channel (Amin and Weiss, 1996). The receptors have a long extracellular domain containing ligand binding sites and several modulatory sites. In the middle of the receptor, GABA gates an ionic channel. Binding of GABA to the receptor induces a conformational change in receptor structure which leads to the opening of the channel.
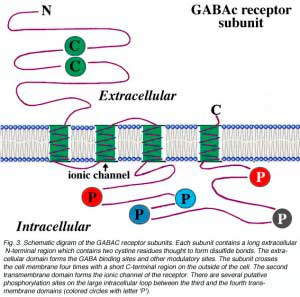
For each subunit forming the receptor, the structure is thought to be that shown in Fig. 3. The subunit contains a long extracellular N-terminal domain which has ligand binding sites, four transmembrane domains, and a large intracellular loop which connects the third and the fourth transmembrane domains. The ionic channel is formed by the second transmembrane domain of each subunit. For the large intracellular loop, there are several putative phosphorylation sites indicated as colored circles with letter ‘P’ in the figure. Phosphorylation of these residues is implicated for modulation of receptor activities. For example, it has been reported that dopamine modulates GABAc receptor activity in both catfish cone-driven horizontal cells and tiger salamander bipolar cell terminals (Dong and Werblin, 1994; Wellis and Werblin, 1995). On rat bipolar cells, the GABAc receptor activities are modulated by protein kinase C (Feigenspan and Bormann, 1994a). The modulation of receptor activities by intracellular second messenger systems is also observed at GABAc receptors expressed on Xenopus oocytes (Kusama et al., 1995). However, the mechanisms for modulation by intracellular second messager systems are yet to be determined. Recently, Filippova and coauthors (1999) provided evidence for GABAc receptor internalization upon phosphorylation of the subunits. In addition, there is evidence that the large intracellular loop of GABAc receptor subunits is involved in the interaction of receptor protein with other intracellular proteins, which may play an important role in the clustering of the receptors on neuronal membranes (Hanley et al., 1999).
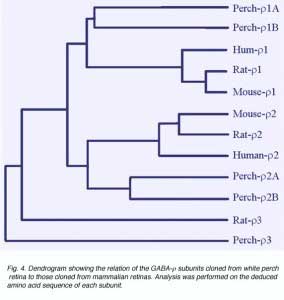
There is much evidence to indicate that GABAc receptors are composed of GABA r subunits. These were first cloned from a human retinal cDNA library (Cutting et al., 1991, 1992). When expressed in Xenopus oocytes, GABA r subunits formed functional homo-oligomeric receptors with properties similar to those of GABAcreceptors in retinal neurons (Shimada 1992). Furthermore, the expression of GABAr subunits has been detected on retinal neurons where GABAcreceptor-mediated responses have been recorded (Qian et al., 1997a; Enz et al., 1995, 1996). In white perch retina, we have now cloned five forms of GABA r subunits (Qian et al., 1997a, 1998). Fig. 4 shows a comparison of white perch GABA r subunits and those cloned from mammalian retinas. The distances between the various connecting elements represent the degree of divergence among subunits. Unlike the mammalian retina, where only one form of r1 and r2 subunits has been identified, in white perch there are two forms of the subunit for each r1 and r2 family. In accordance with their deduced amino acid sequences and the properties of the receptors they formed on Xenopus oocytes, each r1 and r2 family was subdivided into an A and B forms. All white perch GABA r1 and r2 subunits are able to form functional homo-oligomeric receptors when expressed in Xenopus oocytes. GABA elicited responses in these expressed receptors are sustained, bicuculline-insensitive, and are not modulated by either benzodiazepines or barbiturates, features typical of GABAc receptors. Like GABAc receptors on retinal neurons, GABA r receptors also gate chloride channels. However, the receptors expressed by each of the GABA r subunits display unique response properties that distinguish one from the other. For example, the sensitivity of GABA activation and picrotoxin inhibition varies among subunits. In addition, I4AA acts as an antagonist on A-type r receptors, whereas it is a partial agonist on B-type r receptors (Qian et al., 1998).
The waveform of receptor-mediated responses plays an important role in shaping the neuronal signal. Interestingly, the kinetics of the GABA response are also different for the receptors formed by each individual subunits. Current-trace responses to application of 10 mM GABA are illustrated in Fig. 5. These recordings indicate that there are significant differences in the kinetics of the GABA responses from Xenopus oocytes depending on which white perch GABA r subunit is expressed. To quantitate the kinetics of the GABA response, the offset GABA responses (current traces after GABA application is terminated) were replotted on a semi-logarithmic scale, with the amplitudes normalized to their initial values (Fig. 6). In each case, the data were fit by a straight line, indicating that offset responses can be described by a single exponential function. The slope of the line represents the time constant of the decay and shows that the receptors formed by the various r subunits exhibit significant differences in their response kinetics. The average time constants of offset responses elicited from receptors formed by various white perch GABA r subunits are shown in the bar graph in Fig. 6C. There are consistent differences between the response kinetics of the two receptor families and between their subgroups. For example, the responses from r1 receptors were significantly slower than those of r2 receptors. Such difference in the response kinetics among r1 and r2 receptors are determined, in large part, by a single residue at the second transmembrane domain of the subunits (Qian et al., 1999). This dichotomy among r1 and r2 subunits is well conserved in all species where GABA r subunits have been cloned. r1 subunits, which have a proline at the residue site, combine to make a receptor with slower kinetics; whereas r2 subunits, which contain a serine at the residue site, form receptors with faster response rate. Thus, receptors made of human r1 subunits exhibit slower response kinetics than receptors made of r2 subunits (Enz and Cutting, 1999). The kinetic differences among the receptors formed by various GABA r subunits could provide building blocks for the nevous system to construct different types of signal filters, and different types of neuronal signalling in the nervous system.
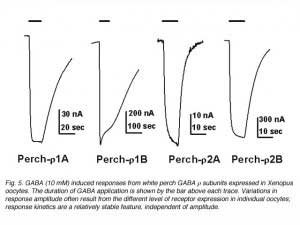 | 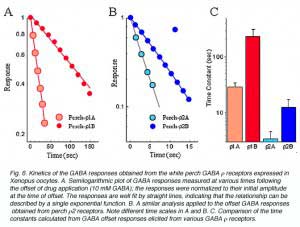 |
Function of GABAc receptors in the retina
Although rod-driven horizontal cells provide an excellent model in which to characterize GABAc receptors in the retina, recent studies indicate GABAc receptors are present on various other types of retinal neurons. GABAc receptor mediated responses have been recorded from cone-driven horizontal cells in catfish (Dong et al., 1994; Kaneda et al., 1997), cone photoreceptors (Picaud et al, 1998), and some types of ganglion cells (Zhang and Slaughter, 1995). GABAc responses are particularly prominent in bipolar cells of every species examined thus far (Feigenspan et al., 1993; Qian and Dowling, 1995; Lukasiewicz et al., 1994; Lukaisiewicz and Wong, 1997; Qian et al., 1997; Nelson et al., 1999), and both immunocytochemistry and in situ hybridization studies indicate GABAc receptors are present on bipolar cells (Qian et al., 1997; Enz et al., 1995, 1996; Koulen et al., 1997). It appears that these receptors play an important role in shaping signal transmission from bipolar cells to third order neurons in the retina.
Fig. 7 illustrates some examples of bipolar cells isolated from white perch retina. These bipolar cells keep their morphology when isolated in culture. They usually have a pear-shaped cell body from which several dendrites and one axon extends. The GABA responses of bipolar cells in white perch retina have both transient and sustained components, indicating both GABAA and GABAc receptors are present as shown in Fig. 8. The transient component can be selectively blocked by the co-application of bicuculline, leaving a more sustained response. Thus, the electrophysiological and pharmacological properties of GABAc receptors on bipolar cells are very similar to those of GABAc receptors on rod-driven horizontal cells (Qian and Dowling 1995; Lukasiewicz et al., 1994; Feigenspan and Bormann, 1994).
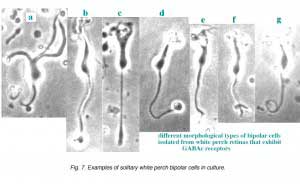 | 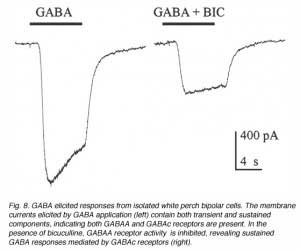 |
Different kinetic properties of GABAA and GABAc receptors suggest that they play different roles in mediating inhibition on bipolar cell terminals (Qian et al., 1997; Lukasiewicz and Shields, 1998). Furthermore, various subtypes of bipolar cells exhibit different proportions of GABAA and GABAc receptors. For example, in the rat retina, there is a clear difference in the contribution of GABAA and GABAc receptors to rod and cone bipolar cells (Euler and Wassle, 1998). In white perch too, different morphological types of bipolar cells exhibit different proportions of GABAc receptor mediated components (Qian and Dowling, 1995). These results strongly suggest that different subtypes of bipolar cell utilize various mixtures of GABAA and GABAc receptors to perform different activities and help create the variety of functional pathways through the retina.
Because of the presence of multiple GABA receptors on retinal neurons, it is sometimes difficult to isolate the contributions of each receptor. Recent studies on ganglion cell responses reveal some interesting features of GABAc receptors in retinal information processing. For example, activation of GABAc receptors leads to more transient light responses in ganglion cells (Dong and Werblin, 1998) and the delayed inhibition mediated by GABAc receptors is thought to play a major role in shaping edge-enhancement of ganglion cell receptive fields (Jacobs and Werblin, 1998). The bipolar cell to ganglion cell synapse is probably heavily influenced by inhibitory amacrine feed forward or feedback synapses and these appear to be via primarily GABAc receptors.
References
- Albrecht BE, Breitenbach U, Stuhmer T, Harvey RJ, Darlison MG. In situ hybridization and reverse transcription-polymerase chain reaction studies on the expression of the GABA(C) receptor rho1- and rho2-subunit genes in avian and rat brain. Eur J Neurosci. 1997;9:2414–2422. [PubMed]
- Amin J, Weiss DS. Insights into the activation mechanism of rho1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits.Proc Biol Sci. 1996;263:273–282. [PubMed]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor rho subunits in rat brain. J Neurochem. 1998;70:899–907. [PubMed]
- Calvo DJ, Vazquez AE, Miledi R. Cationic modulation of r1-type g-aminobutyrate receptors expressed in Xenopus oocytes. Proc Natl Acad Sci U S A.1994;91:12725–12729. [PubMed] [Free Full text in PMC]
- Chang Y, Amin J, Weiss DS. Zinc is a mixed antagonist of homomeric r1 g-aminobutyric acid-activated channels. Mol Pharmacol. 1995;47:595–602.[PubMed]
- Chang Y, Weiss DS. Channel opening locks agonist on to the GABAC receptor. Nat Neurosci. 1999;2:219–225. [PubMed]
- Cutting GR, Lu L, Zoghbi H, O’Hara BF, Kasch LM, Montrose-Rafizader C, Donovan DM, Shimada S, Antonarakis SE, Guggino W, Uhl GR, Kazazian HH. Cloning of the g-aminobutyric acid (GABA) rho1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991;88:2673–2677. [PubMed] [Free Full text in PMC]
- Cutting GR, Curristin S, Zoghbi H, O’Hara B, Selden MF, Uhl GR. Identification of a putative gamma-aminobutyric acid (GABA) receptor subunit rho2 cDNA and colocalization of the genes encoding rho2 (GABRR2) and rho1 (GABRR1) to human chromosome 6q14-q21 and mouse chromosome 4. Genomics.1992;12:801–806. [PubMed]
- Dong CJ, Picaud SA, Werblin FS. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci.1994;14:2648–2658. [PubMed]
- Dong CJ, Werblin FS. Dopamine modulation of GABAC receptor function in an isolated retinal neuron. J Neurophysiol. 1994;71:1258–1260. [PubMed]
- Dong CJ, Werblin FS. Zinc down modulates the GABAc receptor current in cone horizontal cells acutely isolated from the catfish retina. J Neurophysiol.1995;73:916–919. [PubMed]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol.1998;79:2171–2180. [PubMed]
- Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor rho 1 and rho2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. [PubMed]
- Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAC receptor rho subunits in the mammalian retina. J Neurosci.1996;16:4479–4490. [PubMed]
- Enz R, Cutting GR. GABAC receptor rho subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur J Neurosci. 1999;11:41–50. [PubMed]
- Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol.1998;79:1384–1395. [PubMed]
- Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993;361:159–163. [PubMed]
- Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994a;481:325–330. [PubMed] [Free Full text in PMC]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994b;288:97–104.[PubMed]
- Filippova N, Dudley R, Weiss DS. Evidence for phosphorylation-dependent internalization of recombinant human r1 GABAC receptors. J Physiol.1999;518:385–399. [PubMed]
- Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP-1B links GABA(C) receptors to the cytoskeleton at retinal synapses.Nature. 1999;397:66–69. [PubMed]
- Jacobs AL, Werblin FS. Spatio temporal pattern sat the retinal output. J Neurophysiol. 1998;80:447–451. [PubMed]
- Johnston GAR. Multiplicity of GABA receptors. In: Olsen RW, Venter JC, editors. Receptor biochemistry and methodology. Vol. 5. Alan R. Liss, Inc.; 1986. p. 57-71.
- Johnston GAR. GABAc receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996;17:319–323. [PubMed]
- Kaneda M, Mochizuki M, Kaneko A. Modulation of GABAC response by Ca2+ and other divalent cations in horizontal cells of the catfish retina. J Gen Physiol. 1997;110:741–747. [PubMed]
- Koulen P, Brandstätter JH, Kröger S, Enz R, Bormann J, Wässle H. Immunocytochemical localization of the GABA(C) receptor rho subunits in the cat, goldfish, and chicken retina. J Comp Neurol. 1997;380:520–532. [PubMed]
- Kusama T, Sakurai M, Kizawa Y, Uhl GR, Murakami H. GABA rho1 receptor: inhibition by protein kinase C activators. Eur J Pharmacol. 1995;291:431–434. [PubMed]
- Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212.[PubMed]
- Lukasiewicz PD, Wong ROL. GABAC receptors on ferret retinal bipolar cells: a diversity of subtypes in mammals? Vis Neurosci. 1997;14:989–994.[PubMed]
- Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol. 1998;79:3157–3167. [PubMed]
- Morris KD, Moorefield CN, Amin J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuro active steroids. Mol Pharmacol.1999;56:752–759. [PubMed]
- Nelson R, Schaffner AE, Li Y-X, Walton MC. Distribution of GABA(C)-like responses among acutely dissociated rat retinal neurons. Vis Neurosci.1999;16:179–190. [PubMed]
- Pan Z-H, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci. 1995;15:2668–2679. [PubMed]
- Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J Physiol. 1998;513:33–42. [PubMed]
- Polenzani L, Woodward RM, Miledi R. Expression of mammalian g-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991;88:4318–4322. [PubMed] [Free Full text in PMC]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. [PubMed]
- Qian H, Dowling JE. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307.[PubMed]
- Qian H, Dowling JE. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928. [PubMed]
- Qian H, Hyatt G, Schanzer A, Hazra R, Hackam A, Cutting GR, Dowling JE. A comparison of GABAC and rho subunit receptors from the white perch retina. Vis Neurosci. 1997a;14:843–851. [PubMed]
- Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997b;78:2402–2412. [PubMed]
- Qian H, Dowling JE, Ripps H. Molecular and pharmacological properties of GABA-rho subunits from white perch retina. J Neurobiol. 1998;37:305–320.[PubMed]
- Qian H, Dowling JE, Ripps H. A single amino acid in the second transmembrane domain of GABA r subunits is a determinant of the response kinetics of GABAC receptors. J Neurobiol. 1999;40:67–76. [PubMed]
- Ragozzino D, Woodward RM, Murata Y, Eusebi F, Overman LE, Miledi R. Design and in vitro pharmacology of a selective gamma-aminobutyric acid C receptor antagonist. Mol Pharmacol. 1996;50:1024–1030. [PubMed]
- Shimada S, Cutting G, Uhl GR. g-Aminobutyric acid A or C receptor? g-Aminobutyric acid r1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive g-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992;41:683–687. [PubMed]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. [PubMed]
- Wang TL, Hackam A, Guggino WB, Cutting GR. A single histidine residue is essential for zinc inhibition of GABA rho 1 receptors. J Neurosci.1995;15:7684–7691. [PubMed]
- Wegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saarma M, Reeben M. Distribution of GABA receptor rho subunit transcripts in the rat brain. Eur J Neurosci. 1998;10:350–357. [PubMed]
- Wellis DP, Werblin FS. Dopamine modulates GABAc receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J Neurosci. 1995;15:4748–4761. [PubMed]
- Woodward RM, Polenzani L, Miledi R. Effects of steroids on g-aminobutyric acid receptors expressed in Xenopusoocytes by poly(A)+ RNA from mammalian brain and retina. Mol Pharmacol. 1992a;41:89–103. [PubMed]
- Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive g-aminobutyric acid receptors expressed in Xenopus oocytes I. effects of Cl- channel inhibitors. Mol Pharmacol. 1992b;42:165–173. [PubMed]
- Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive (r-like) g-aminobutyric acid receptors expressed in Xenopus oocytes. II. pharmacology of g-aminobutyric acid A and g-aminobutyric acid B receptor agonists and antagonists. Mol Pharmacol. 1993;43:609–625.[PubMed]
- Zhang D, Pan ZH, Zhang X, Brideau AD, Lipton SA. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxin in channel block. Proc Natl Acad Sci U S A. 1995;92:11756–11760. [PubMed] [Free Full text in PMC]
- Zhang J, Slaughter MM. Perferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592.[PubMed]
The author Dr. Haohua Qianwas born in Jiangsu, China. He received his B.A. in Biology from Nanjing University (1982), M.S. in Neurobiology from Shanghai Institute of Physiology (1985), and Ph.D. in Anatomy and Cell Biology from University of Illinois at Chicago (1991). He is currently an Assistant Professor of Neurosciences in the Department of Ophthalmology and Visual Sciences at the University of Illinois at Chicago. During his postdoctoral studies with Dr. John E. Dowling at Harvard University, he characterized a new type of GABA receptor, the GABAc r receptor, on retinal neurons. He is currently continuing these studies on the molecular structure and physiological functions of GABAc receptors in the vertebrate retina.