1. Introduction
The crystalline lens contributes approximately +15 to +20 diopters (D) to the refractive power of the human eye in its non-accommodative state. A further 43 diopters is provided by the cornea (see chapter “Facts and Figures”). So when cataracts occur (almost inevitably in the ageing eye) the cataractous lens must be removed to restore a clear image passage through to the retina. After removal of an opacified crystalline lens in cataract surgery, the refractive power of a normal clear lens needs to be restored.1 In modern cataract surgery, a central opening of approximately 5.5 mm is made to the anterior capsule of the crystalline lens (capsulorhexis) and the opacified contents within the capsular bag are then removed by phacoemulsification and irrigation/aspiration. An artificial intraocular lens (IOL) is then placed within the intact capsular bag. However, if an IOL is not implanted inside the eye, the refractive power of the removed cataractous crystalline lens can still be restored by contact lenses or spectacles.2 And that is what happened in the old days. The offending cataractous lens was removed and the patient was given a pair of thick spectacles. An eye with a natural crystalline lens is called a phakic eye and without a natural crystalline lens but with an IOL is called a pseudophakic eye.
During World War II, Sir Harold Ridley, an ophthalmologist in London saw Royal Air Force casualties with eye injuries, and observed that when splinters of acrylic plastic from aircraft cockpit canopies became lodged in their eyes, this did not trigger inflammatory rejection as did glass splinters. This led him to propose the use of artificial lenses made of Perspex (polymethyl methacrylate – PMMA), to treat cataracts. On 29 November 1949 at St Thomas’ Hospital, Ridley achieved the first implant of an IOL, although it was not until 8 February 1950 that he left an IOL permanently in place in an eye.2
Cataract surgery with IOL implantation has now become the most prevalent eye operation in the United States with more than 3 million procedures performed in 2006 alone.3 For patients with dense cataracts this surgery has become a “miracle” restoring clear sight again. A variety of new artificial IOL designs, manufactured from different biomaterials, are continuously being made available to cataract surgeons nowadays. In this chapter we provide an overview of the characteristics of IOLs used in cataract surgery, as well as possible complications associated with their use.
2. IOL materials
When examining IOL materials, it can be broken down into four general types. The initial breakdown can distinguish between groups of lenses made of acrylic or silicone material. When describing acrylic lenses, composed of acrylic acid, they can be further broken down into non-foldable or foldable types, referring to their flexibility. The non-foldable lenses are made up of Polymethyl methacrylate (PMMA) material. Within the foldable lenses, they can be further broken up into hydrophobic acrylic or hydrophilic acrylic materials. Silicone lenses are the last group of materials, and all silicone lenses are foldable. The utility of flexible, foldable materials is that the lenses can then be inserted (with forceps) or injected (with injector systems) into the eye via small incisions, generally 3.0 mm wide or less.4-7
Polymethyl methacrylate (PMMA) lenses were the first lenses to be used in the human eye (in 1949). They were rigid and required a large incision to be placed in the eye. As cataract surgery technology has advanced, they are now the least used IOL material in developed countries.5
The decrease in usage of PMMA lenses was primarily attributed to the advent of small incision cataract surgery techniques utilizing ultrasound phacoemulsification, and the increase in popularity of the foldable acrylic lenses. Foldable acrylic lenses can either be composed of hydrophobic acrylic or hydrophilic acrylic material. Generally, hydrophobic acrylic lenses absorb very little water (<1%). On the other hand, hydrophilic acrylic lenses absorb significantly higher amounts (18-38%). Each currently available foldable acrylic lens design is manufactured from a different copolymer acrylic, resulting in a different refractive index, glass transition temperature (the polymer is flexible above this temperature and rigid below), water content, mechanical properties, etc. The flexibility and high water content allows for certain hydrophilic acrylic lenses to be inserted through very small incisions, approximately 1.8 mm, in microincision cataract surgery.4
Silicone lenses were the first foldable IOLs available on the market. They are composed of a polyorganosilone backbone and have refractive indexes (e.g., 1.46) that are usually lower than those of acrylic lenses (e.g., 1.55). As a result, they are thicker lenses than their acrylic counterparts.4,7
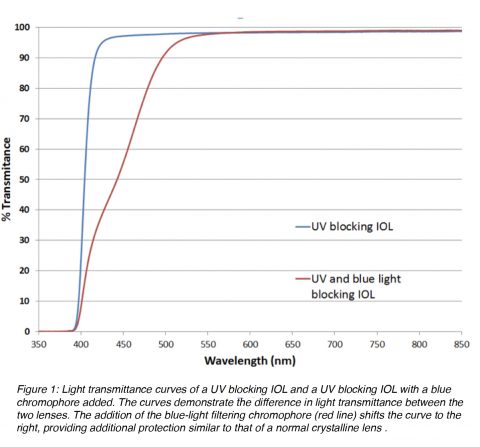
Ultraviolet (UV)-absorbing compounds (chromophores) are another important element of the IOL optic component. They protect the retina from UV radiation in the 300-400 nm range, a feature normally provided by the normal crystalline lens. Yellow hydrophobic acrylic IOLs containing a blue light-filtering chromophore (different from the standard chromophore for protection against UV radiation) are also available on the market (Figure 1). The addition of a covalently bonded yellow dye results in an IOL with an UV/visible light transmittance curve that mimics the protection provided by the normal crystalline lens in adult humans. There is indirect evidence showing that this may result in a reduction of the risk for development and progression of macular degeneration. But this remains a controversial issue.4
3. IOL designs
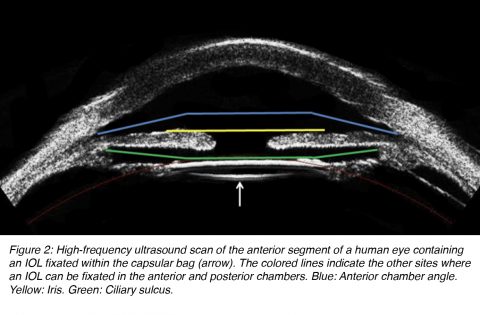
In modern cataract surgery, an IOL is ideally fixated within the capsular bag. If the capsular bag is not intact at the end of surgery, an IOL can still be fixated at other sites. However, the design of the lens has to be adapted for implantation in each different site of fixation. IOL designs can be categorized in different ways. According to the fixation site, IOLs may be implanted in the anterior or posterior chamber of the eye. Anterior chamber lenses can be placed in the anterior chamber angle or fixated to the iris. Posterior chamber lenses can be fixated within the capsular bag or the ciliary sulcus (Figure 2).5
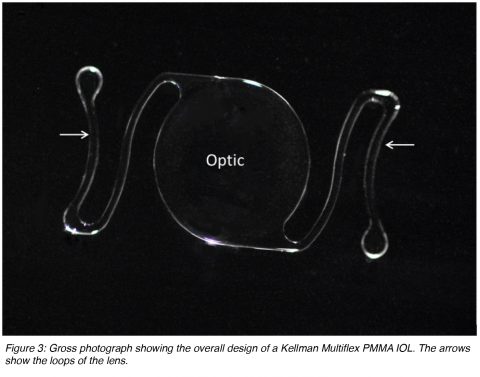
IOLs can also be described as single-piece (the entire lens is manufactured from the same material) or multi-piece (generally described as 3-piece lenses, the optic lens from one source and the loops manufactured from a different material). Anterior chamber lenses placed in the angle that are currently available on the market are based on the Kelman Multiflex design (Figure 3). These are single-piece lenses made of PMMA with open loops. The overall diameter of these lenses generally ranges between 12 and 14 mm. The size is chosen according to the anterior chamber diameter of the eye receiving the implant. There is an angulation between the optic component and the loops that makes the optic more anteriorly located.
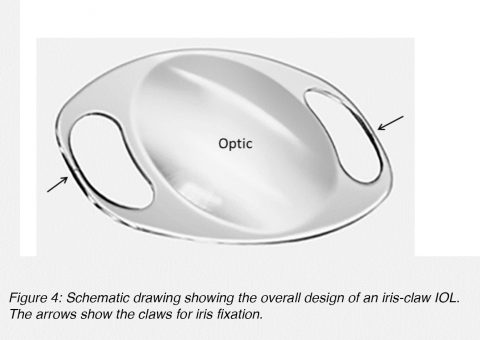
Anterior chamber lenses fixated to the iris are generally based on the iris-claw design (Figure 4). These lenses are also single-piece lenses made of PMMA, with an overall diameter of approximately 8.5 mm. The fixation of the lens to the iris stroma is obtained through small claws situated on both sides of the optic component (Figure 4, arrows).5
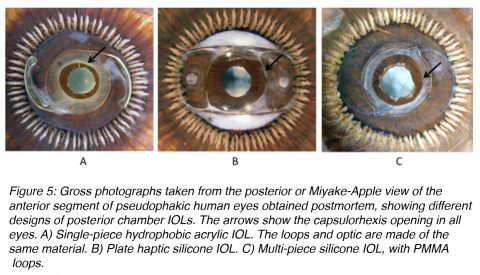
Posterior chamber lenses designed for fixation within the lens capsular bag come in a large variety of designs (Figure 5). They can be single-piece or multi-piece lenses. Single-piece lenses may be open loop designs or overall plate lenses. Overall plate lenses can incorporate a number of different fixation elements, generally represented by small open or closed loops (otherwise known as haptics). Different materials may be used for the manufacture of the haptic component of three-piece lenses, including PMMA, polypropylene (Prolene), polyimide (Elastimide) and poly(vinylidene) fluoride (PVDF).5,8 Fixation of flexible-looped IOLs is achieved by exerting centripetal pressure on the surrounding ocular tissues. During IOL insertion, the loops are bent centrally and re-expand as the forces exerted on the loops are released. The two factors that contribute to the ability of IOL loops to maintain their original symmetrical configuration are loop rigidity, the resistance of the haptic to external forces that act to bend the loops centrally, and loop memory, the ability of the loops to re-expand laterally to their original size and configuration. Ideally, IOL loops should have enough flexibility to allow easy insertion and accommodation to the circular shape of the eye. Furthermore, they should prevent damage to supporting structures during insertion and in the postoperative period. They should also have appropriate rigidity to resist external forces, such as capsular bag contraction from capsular fibrosis.8
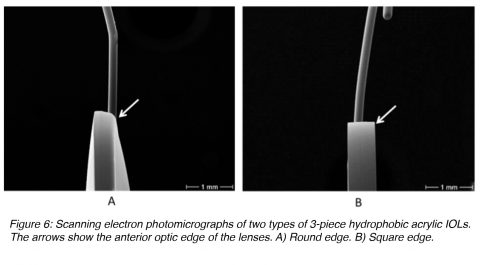
There has to be some angle between the optic component and the loops of 3-piece lenses manufactured for implantation in the posterior chamber of the eye (capsular bag or ciliary sulcus (Figure 2), to make the optic component more posteriorly located. If the capsular bag is not intact at the end of the surgery, a posterior chamber lens may be fixated in the ciliary sulcus, in front of the anterior lens capsule if there is enough capsular support present. Clinical evidence suggests that IOLs fixated in the sulcus should have a smooth rounded anterior optic edge and thin haptics (Figure 6A). This will minimize their interaction with the posterior iris surface and prevent complications such as pigmentary dispersion syndrome.9,10 The diameter of ciliary sulcus fixated lenses must be sufficiently large to prevent IOL displacement from the center (at least 13 mm). If the residual capsular support is insufficient, a posterior chamber lens can still be fixated in the ciliary sulcus by suturing the IOL loops to the sclera. There are also techniques to suture the loops of these lenses to the iris.
4. Biocompatibility
Biocompatibility is one of the most important prerequisites of an IOL. It is important to note that IOLs are progressively being used at earlier stages of life. As a result, it is vital that they are able to stay in the intraocular environment and remain transparent without complications for many years.
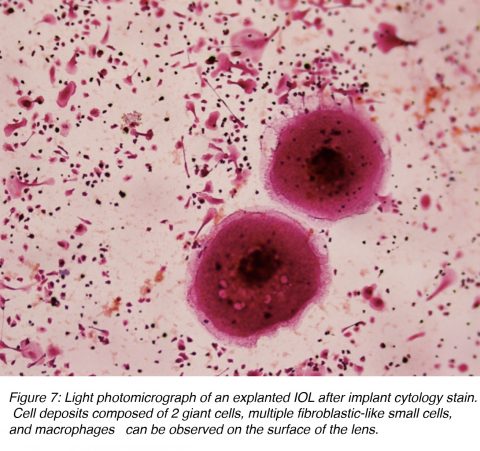
The term uveal biocompatibility is generally used to describe the inflammatory response of the eye towards the artificial IOL (foreign-body reaction).11-13 Cataract surgery with implantation of an IOL results in a breakdown of the blood-aqueous barrier, causing a release of proteins and cells into the anterior chamber. Protein adsorption on the surface of the IOL is the first phenomenon observed and it influences the subsequent cell interaction between the interface material and tissue. The complement system is activated by the alternative pathway, which results in the attraction of polymorphonuclear leukocytes and monocytes. These cells are the beginning of the macrophages and giant cells that constitute a foreign-body reaction against the IOL (Figure 7). Inflammatory cell deposits are a normal occurrence on the lens surface for up to 1 year after surgery. This consists of two distinct processes: a response with small round and fibroblast-like cells, which peaks by 1 month, and a later giant cell response, which peaks at 3 months. Giant cells then degenerate and detach from the IOL surface, leaving only an acellular proteinaceous membrane usually surrounding the IOL, which isolates it from the surrounding ocular tissues.11-13
One of us (Liliana Werner) reviewed several published studies that analyzed postoperative flare values and found that there were no clinically relevant differences between different biomaterials. She further went on to note that there was variation in the intensity and duration of each cellular inflammatory response (small cells or giant cells) amongst different biomaterials. However, the cellular reaction was low grade and therefore clinically insignificant.4
Capsular biocompatibility
When discussing capsular biocompatibility, the relationship of an IOL with remaining lens epithelial cells (LECs) within the capsular bag after cataract surgery,11 it is important to understand the epithelium of the normal crystalline lens. This consists of a sheet of anterior epithelial cells (“A” cells) that are in continuity with the cells of the equatorial lens bow (“E” cells) (see Chapter on “Lens and Cataract” in Webvision, by Alliancy and Mamalis). The two cell types differ in function, growth patterns, and pathologic processes. E cells are germinal cells that undergo mitosis and form new lens fibers, whereas A cells do not. When disturbed by pathologic processes (or surgery), A cells do not have the tendency to migrate whereas E cells tend to migrate posteriorly toward the posterior lens capsule. A cells have a tendency to transform into fibrous-like tissue (fibrous metaplasia). In contrast, E cells typically enlarge into bladder-like cells referred to as Elschnig pearls (see accompanying chapter). These cells’ differences become particularly important when examining different forms of postoperative opacification of the capsular bag, such as anterior capsule opacification and posterior capsule opacification.14
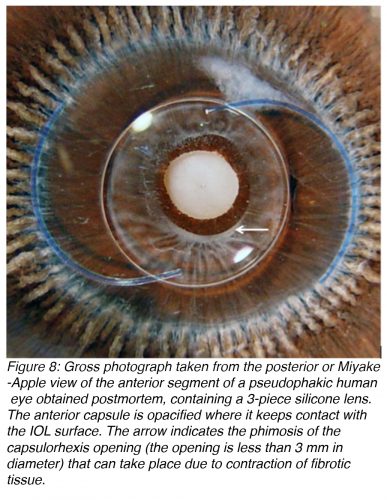
Anterior capsule opacification (ACO) occurs as a result of the anterior surface of an IOL optic coming in contact with the posterior aspect of the residual anterior lens capsule (Figure 8). This causes the A cells of the lens to undergo fibrous metaplasia and results in capsule opacification.15-16 ACO is essentially a fibrotic reaction. This is of concern due to the development of the new accommodating IOLs, which are usually designed to move within the capsular bag during accommodation. The resulting fibrosis may restrict these movements.
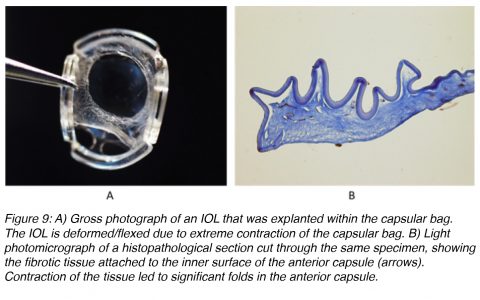
Contraction of fibrotic tissue may also cause capsulorhexis phimosis (which is the decrease in diameter of the capsulorhexis opening to less than 3 mm), which may lead to the IOL being thrown off center and possibly even deformation of IOLs that are very thin and flexible (Figure 9).17-19 ACO may be prevented by surgical polishing of the inner surface of the anterior capsule during surgery that results in the removal of residual A cells. Also, ACO can be prevented by implantation of an IOL that does not maintain a large area of contact with the inner surface of the anterior capsule.20
Whereas ACO is more of an effect of the residual A cells, posterior capsule opacification (PCO) has fibrotic and regeneratory or “pearl” components.21-24 PCO is the most common postoperative complication of cataract surgery, occurring at a rate of between 3-50% in the first five postoperative years. It occurs because of residual LECs that remain within the capsular bag following the removal of the crystalline lens during cataract surgery. These cells can proliferate and migrate across the posterior capsule where they may cause wrinkling and complete opacification of this structure. As a result, the patient will describe distortion, decrease of vision, and glare. The LECs have higher proliferative capacity in younger patients; therefore, the incidence of PCO formation is higher.21-24
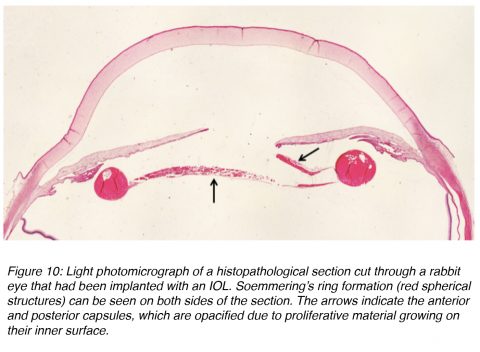
Regeneratory PCO is attributed more to the action of residual E Cells.24 These cells are responsible for formation of a Soemmering’s ring, defined as a doughnut-shaped lesion composed of retained/regenerated cortex and cells that may form following any disruption of the anterior lens capsule. For practical purposes, it is useful to consider this lesion as the basic precursor of classic PCO, especially the “pearl” form (Figure 10).23
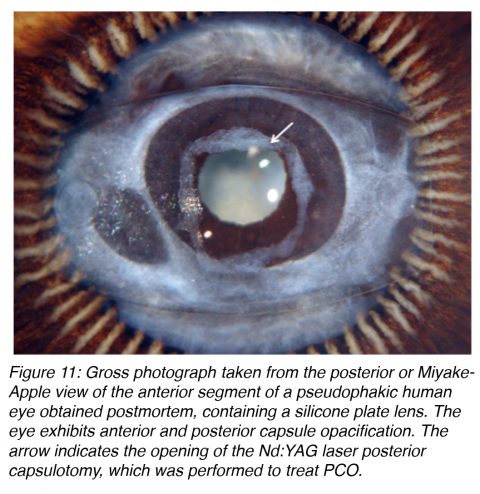
The treatment of PCO is typically neodymium:YAG (Nd:YAG) laser posterior capsulotomy (Figure 11). This is a simple procedure in most cases, but it is not without risks. Complications include IOL damage, IOL subluxation or dislocation, retinal detachment, or secondary glaucoma. Therefore prevention of this complication is important, not only because of the risks associated with its treatment, but also because of the costs involved in the procedure.14,23
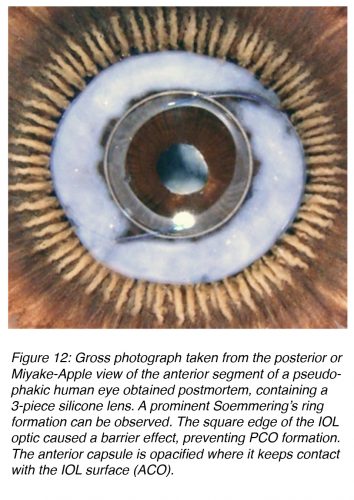
While basic research on the effective mechanism for PCO eradication evolves, the practical surgeon can already apply some principles to prevent it. Studies in our laboratory and clinical studies at other centers resulted in the definition of three surgery-related factors that help in the prevention of PCO: 1) Hydrodissection-enhanced cortical clean-up, 2) In-the-bag IOL fixation, and 3) Performance of a capsulorhexis slightly smaller than the diameter of the IOL optic (for a shrink-wrap effect of the IOL by the capsule). The same studies helped in the definition of three IOL-related factors for PCO prevention: 1) Use of a biocompatible IOL to reduce cellular proliferation, 2) Enhancement of the contact between the IOL optic and the posterior capsule, and 3) An IOL with a square, truncated posterior optic edge. The last two IOL factors enhance the barrier effect of the IOL optic against material coming from the Soemmering’s ring, a precursor for PCO (Figure 12).14,23,25-30
5. Causes of IOL opacification
Postoperative opacification of the IOL optic is a rare complication, but it can ultimately lead to the necessity of explantation and exchange of the IOL because of decreased visual function.31 Postoperative calcification may be observed with some hydrophilic acrylic lenses. Additionally, silicone lenses can also suffer from calcification in certain conditions (history of asteroid hyalosis, which is a condition where there are many calcium deposits floating in the vitreous). Some PMMA lenses may exhibit a specific form of degeneration called snowflake degeneration.32
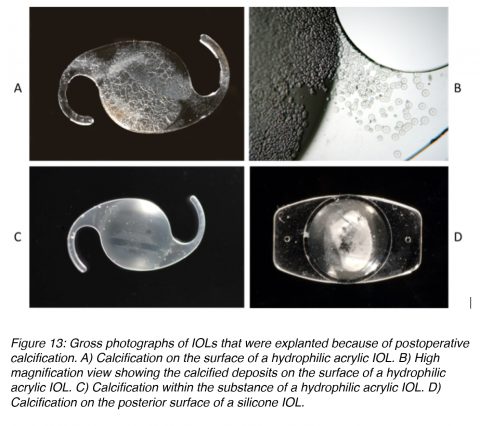
Calcification of hydrophilic acrylic lenses appears to be caused by many problems. Factors related to IOL manufacturing, IOL packaging, surgical techniques and adjuvants, and the patient’s metabolic condition are possible causes. As the exact combination of factors and sequence of events ultimately leading to calcification of the lenses is still unsure, continuous research on this complication is ongoing.32,33 The majority of the studies on calcified hydrophilic acrylic lenses describe explantation during the second postoperative year or earlier. In some cases the deposits causing the opacification are found on the optical surface or subsurface of the lenses (Figures 13A and 13B).34,35 However, in other cases they are predominantly found within the optic substance (Figure 13C).36,37
In cases of calcification of silicone lenses in eyes with asteroid hyalosis, studies demonstrated deposits only on the posterior optic surface of the lenses (Figure 13D). The deposits can be partially removed with Nd:YAG laser, but there is a re-accumulation after the procedure because the asteroid bodies are rich in calcium/phosphate. Asteroid hyalosis is a degenerative condition in which hydroxyapatite (calcium/phosphate) bodies form in the vitreous of the eye. The opacification formed on the silicone IOLs is unique because this type of calcification had not been reported in patients without asteroid hyalosis.38-41
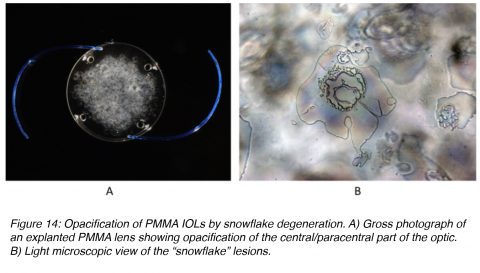
Snowflake degeneration is a slowly progressive opacification that has been observed in three-piece PMMA lenses implanted between the early 1980s and the mid 1990s. They were generally manufactured by a technique called injection molding. Snowflake degeneration is the result of PMMA degradation caused by long-term UV light exposure, not calcium deposition. The degree of optic opacification on the majority of the PMMA lenses requiring explantation may take 10-20 years to develop. The intraoptic spherical lesions (resembling snowflakes) observed in these cases are thought to correspond to sites of degenerated PMMA material. The lesions are clustered around the center of the optic as opposed to the periphery, which could be protected from UV exposure by the iris (Figure 14).42-46
6. Conclusion
In summary, cataract surgery is the most prevalent operation in the United States, and likely worldwide, resulting in implantation of large numbers of artificial IOLs every year. A variety of new IOL designs, manufactured from different biomaterials, are continuously being made available to cataract surgeons to replace the refractive power of the removed opacified crystalline lens. The biomaterials used in IOL manufacture are basically divided into acrylic (rigid and foldable) and silicone (foldable). Posterior capsule opacification PCO remains the most common postoperative complication of cataract surgery. Its incidence has decreased over the past few decades as the understanding of its pathogenesis has become available. Advances in surgical technique, IOL design, and materials have all contributed to the gradual decline in PCO incidence. Although relatively rare, explantation of an IOL may be required due to problems related to postoperative opacification of the IOL, among others. With the increasing number of new lenses in the market every year, constant vigilance regarding overall IOL biocompatibility is warranted and ongoing in our laboratory.
References
- Normal Crystalline Lens. American Academy of Ophthalmology, ONE Network. http://www.aao.org/bcscsnippetdetail.aspx?id=f38d473f-c836-4fe6-8555-20d34ce19816
- Buratto L, Werner L, Zanini M, Apple DJ, Editors. Phacoemulsification: Principles and Techniques (Second Edition). Slack Inc., Thorofare, NJ, USA, 2003.
- Rush SW, Gerald AE, Smith JC, Rush JA, Rush RB. Prospective analysis of outcomes and economic factors of same-day bilateral cataract surgery in the United States. J Cataract Refract Surg. 2015; 41:732-739. [PubMed]
- Werner L. Biocompatibility of intraocular lens materials. Curr Opin Ophthalmol 2008; 19(1): 41-49. [PubMed]
- Werner L. Lentes Intraoculares: Materiais e Desenhos. In: Ambrósio Jr. R, Crema A, eds. Tratado Brasileiro de Catarata e Cirurgia Refrativa. Grupo Editorial Nacional, Rio de Janeiro, RJ, Brazil, 2014; chapter 47, pp. 265-271.
- Ness PJ, Werner L, Maddula S, Davis D, Zaugg B, Stringham J, Burrow M, Yeh O. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: capsular bag opacification and sites of square-edged barrier breach. J Cataract Refract Surg 2011; 37:923-930. [PubMed]
- Maddula S, Werner L, Ness PJ, Davis D, Zaugg B, Stringham J, Burrow M, Yeh O. Pathology of 157 human cadaver eyes with round-edged or modern square-edged silicone intraocular lenses: analyses of capsule bag opacification. J Cataract Refract Surg 2011; 37:740-748. [PubMed]
- Izak AM, Werner L, Apple DJ, Macky TA, Trivedi RH, Pandey SK. Loop memory of different haptic materials used in the manufacture of posterior chamber intraocular lenses. J Cataract Refract Surg 2002; 28:1229-1235. [PubMed]
- Kirk KR, Werner L, Jaber R, Strenk S, Strenk L, Mamalis N. Pathologic assessment of complications with asymmetric or sulcus fixation of square-edged hydrophobic acrylic intraocular lenses. Ophthalmology 2012; 119:907-913. [PubMed]
- Ollerton A, Werner L, Strenk S, Strenk L, Leishman L, Bodnar Z, Kirk KR, Michelson J, Mamalis N. Pathologic comparison of asymmetric or sulcus fixation of 3-piece intraocular lenses with square versus round anterior optic edges. Ophthalmology 2013; 120:1580-1587. [PubMed]
- Amon M. Biocompatibility of intraocular lenses (letter) J Cataract Refract Surg. 2001;27:178–179. [PubMed]
- Obstbaum SA. The Binkhorst Medal Lecture. Biologic relationship between poly(methyl methacrylate) intraocular lenses and uveal tissue. J Cataract Refract Surg 1992; 219–231. [PubMed]
- Hollick EJ, Spalton DJ, Ursell PG, Pande MV. Biocompatibility of poly(methyl methacrylate), silicone, and AcrySof intraocular lenses: randomized comparison of the cellular reaction on the anterior lens surface. J Cataract Refract Surg 1998; 24:361–366. [PubMed]
- Apple DJ, Werner L. Complications of cataract and refractive surgery: A clinicopathological documentation. Trans Am Ophthalmol Soc 2001; 99:95-109. [PubMed]
- Werner L, Pandey SK, Escobar-Gomez M, et al. Anterior capsule opacification: A histopathological study comparing different IOL styles. Ophthalmology 2000; 107:463-471. [PubMed]
- Werner L, Pandey SK, Apple DJ, et al. Anterior capsule opacification: correlation of pathological findings with clinical sequelae. Ophthalmology 2001; 108:1675-1681. [PubMed]
- Zaugg B, Werner L, Neuhann T, Burrow M, Davis D, Mamalis N, Tetz M. Clinicopathologic correlation of capsulorhexis phimosis with anterior flexing of single-piece hydrophilic acrylic intraocular lens haptics. J Cataract Refract Surg 2010; 36:1605-1609. [PubMed]
- Epstein RH, Liu ET, Werner L, Kohnen T, Kaproth OK, Mamalis N. Capsulorhexis phimosis with anterior flexing of an accommodating IOL: Case report and histopathological analyses. J Cataract Refract Surg 2014; 40:148-152. [PubMed]
- Kramer GD, Werner L, Neuhann T, Tetz M, Mamalis N. Anterior haptic flexing and in-the-bag subluxation of an accommodating intraocular lens due to excessive capsular bag contraction. J Cataract Refract Surg 2015; 41(9):2010-2015. [PubMed]
- Kramer GD, Werner L, Mamalis N. Prevention of postoperative capsular bag opacification using intraocular lenses and endocapsular devices maintaining an open or expanded capsular bag. J Cataract Refract Surg 2016; 42:469-484. [PubMed]
- Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsular opacification. Major review. Surv Ophthalmol 1992; 37:73-116. [PubMed]
- Saika S, Werner L, Lovicu FJ, Editors. Lens Epithelium and Posterior Capsular Opacification. Springer Japan, Tokyo, Japan, 2014.
- Werner L. The Lens, Secondary Cataract (Chapter 5.16). In: Yanoff M, Ducker JS, eds. Ophthalmology (Fourth Edition). Philadelphia, PA: Elsevier Saunders, 2013.
- Georgopoulos M, Findl O, Menapace R, Buehl W, Wirtitsch M, Rainer G. Influence of intraocular lens material on regeneratory posterior capsule opacification after neodymium:YAG laser capsulotomy. J Cataract Refract Surg 2003; 29(8):1560-1565. [PubMed]
- Nishi O, Yamamoto N, Nishi K, Nishi Y. Contact inhibition of migrating lens epithelial cells at the capsular bend created by a sharp-edged intraocular lens after cataract surgery. J Cataract Refract Surg 2007; 33:1065–1070. [PubMed]
- Nishi O, Nishi K, Sakanishi K. Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthalmic Surg Lasers 1998; 29:587–594. [PubMed]
- Boyce JF, Bhermi GS, Spalton DJ, El-Osta AR. Mathematical modeling of the forces between an intraocular lens and the capsule. J Cataract Refract Surg 2002; 28:1853–1859. [PubMed]
- Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses. Microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg 2008; 34:310-317. [PubMed]
- Werner L, Tetz M, Feldmann I, Bücker M. Evaluating and defining the sharpness of intraocular lenses: microedge structure of commercially available square-edged hydrophilic intraocular lenses. J Cataract Refract Surg 2009; 35:556-566. [PubMed]
- Werner L, Mamalis N, Pandey SK, et al. Posterior capsule opacification in rabbit eyes implanted with hydrophilic acrylic intraocular lenses with enhanced square edge. J Cataract Refract Surg 2004; 30:2403-2409. [PubMed]
- Mamalis N, Brubaker J, Davis D, Espandar L, Werner L. Complications of foldable intraocular lenses requiring explantation or secondary intervention–2007 survey update. J Cataract Refract Surg 2008; 34:1584-1591. [PubMed]
- Werner L. Causes of intraocular lens opacification or discoloration. J Cataract Refract Surg 2007; 33:713-726. [PubMed]
- Barra D, Werner L, Costa JLP, Morris C, Ribeiro T, Ventura BV, Dornelles F. Light scattering and light transmittance in a series of calcified single-piece hydrophilic acrylic intraocular lenses of the same design. J Cataract Refract Surg 2014; 40:121-128. [PubMed]
- Werner L, Apple DJ, Escobar-Gomez M, Öhrström A, Crayford BB, Bianchi R, Pandey SK. Postoperative deposition of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology 2000; 107:2179-2185. [PubMed]
- Neuhann IM, Werner L, Izak AM, Pandey SK, Kleinmann G, Mamalis N, Neuhann TF, Apple DJ. Late postoperative opacification of a hydrophilic acrylic (hydrogel) intraocular lens: A clinicopathological analysis of 106 explants. Ophthalmology 2004; 111:2094-2101. [PubMed]
- Werner L, Apple DJ, Kaskaloglu M, Pandey SK. Dense opacification of the optical component of a hydrophilic acrylic intraocular lens: a clinicopathological analysis of 9 explanted lenses. J Cataract Refract Surg 2001; 27:1485-1492. [PubMed]
- Werner L, Hunter B, Stevens S, Chew JJL, Mamalis N. Role of silicon contamination on calcification of hydrophilic acrylic intraocular lenses. Am J Ophthalmol 2006; 141:35-43. [PubMed]
- Foot L, Werner L, Gills JP, Shoemaker DW, Phillips PS, Mamalis N, Olson RJ, Apple DJ. Surface calcification of silicone plate intraocular lenses in patients with asteroid hyalosis. Am J Ophthalmol 2004; 137:979-987. [PubMed]
- Werner L, Kollarits CR, Mamalis N, Olson RJ. Surface calcification of a three-piece silicone intraocular lens in a patient with asteroid hyalosis: A clinicopathologic case report. Ophthalmology 2005; 112:447-452. [PubMed]
- Stringham J, Werner L, Monson B, Theodosis R, Mamalis N. Calcification of different designs of silicone intraocular lenses in eyes with asteroid hyalosis. Ophthalmology 2010; 117:1486-1492. [PubMed]
- Espandar L, Mukherjee N, Werner L, Mamalis N, Kim T. Diagnosis and management of opacified silicone intraocular lenses in patients with asteroid hyalosis. J Cataract Refract Surg 2015; 41:222-225. [PubMed]
- Apple DJ, Peng Q, Arthur SN, Werner L, Merritt JH, Vargas LG, Hoddinott DS, Escobar-Gomez M, Schmidbauer JM. Snowflake degeneration of polymethyl methacrylate posterior chamber intraocular lens optic material: a newly described clinical condition caused by unexpected late opacification of polymethyl methacrylate. Ophthalmology 2002; 109:1666-1675. [PubMed]
- Dahle N, Werner L, Fry L, Mamalis N. Localized, central optic snowflake degeneration of a PMMA intraocular lens: Clinical report with pathological correlation. Arch Ophthalmol 2006; 124:1350-1353. [PubMed]
- Michelson J, Werner L, Ollerton A, Leishman L, Bodnar Z. Light scattering and light transmittance in intraocular lenses explanted because of optic opacification. J Cataract Refract Surg 2012; 38:1476-1485. [PubMed]
- Werner L, Michelson J, Ollerton A, Leishman L, Bodnar Z. Anterior segment optical coherence tomography in the assessment of postoperative intraocular lens optic changes. J Cataract Refract Surg 2012; 38:1077-1085. [PubMed]
- Werner L, Stover JC, Schwiegerling J, Das KK. Effects of intraocular lens opacification on light scatter, stray light, and overall optical quality/performance. Invest Ophthalmol Vis Sci 2016; 57(7):3239-3247. [PubMed]
About the Authors

Liliana Werner, MD, PhD is a Tenured Professor of Ophthalmology and Visual Sciences, and Co-Director of the Intermountain Ocular Research Center, at the John A. Moran Eye Center, University of Utah. She has an MD/Ophthalmology degree from Brazil, and a PhD degree (Biomaterials) from France (Université Paris V, René Descartes). Dr. Werner’s research is centered on the interaction between ocular tissues and different intraocular lens designs, materials and surface modifications. These include intraocular lenses implanted after cataract surgery, and also phakic lenses for refractive surgery and ophthalmic implantable devices in general. She has authored more than 300 peer-reviewed publications and book chapters on the subject, co-edited 3 books, and received numerous awards in international meetings for scientific presentations, videos and posters. She has also been a guest speaker in different international meetings in at least 20 countries, and is a consultant for different companies manufacturing IOLs and other ocular biodevices. Liliana can be reached at liliana.werner@hsc.utah.edu

Jason Nguyen, MD is an Ocular Pathology and Research Fellow at the Intermountain Ocular Research Center, Moran Eye Center, University of Utah. He graduated with a MD degree from the University of Oklahoma College of Medicine and will be starting his Ophthalmology residency at West Virginia University in July 2018.