1. General morphology
Ganglion cells are the final output neurons of the vertebrate retina. The ganglion cell collects the electrical messages concerning the visual signal from the two layers of nerve cells preceding it in the retinal wiring scheme. A great deal of preprocessing has been accomplished by the neurons of the vertical pathways (photoreceptor to bipolar to ganglion cell chain), and by the lateral pathways (photoreceptor to horizontal cell to bipolar to amacrine to ganglion cell chain) before presentation to the ganglion cell and so it represents the ultimate signaller to the brain of retinal information. Ganglion cells are larger on average than most preceding retinal interneurons and have large diameter axons capable of passing the electrical signal, in the form of transient spike trains, to the retinal recipient areas of the brain many millimeters or centimeters distant from the retina. The optic nerve collects all the axons of the ganglion cells and this bundle of more than a million fibers (in man at least) then passes information to the next relay station in the brain for sorting and integrating into further information processing channels.
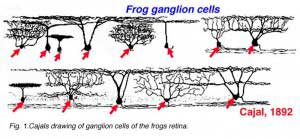
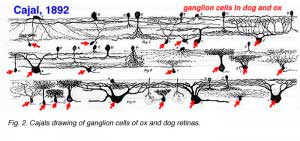
Cajal in his monumental work on Golgi staining of the vertebrate retina was able to classify many different varieties of ganglion cell based on form (dendritic morphology), extent (cell body and dendritic tree size), and number of sublayers in which they arborize (stratification levels in the inner plexiform layer). He considered the retina to be remarkably uniform across all vertebrates differing only in respect to rod and cone specializations for the visual sense of the animal. Looking at Cajals (1892) drawings of dog ganglion cells as compared frog ganglion cell for example the former seem simpler in form than the latter cells (see Figs. 1 and 2 above) but we actually now know that there is a common evolutionary path taken by different ganglion cell types so that different morphological and functional classes are similar throughout the species. For example the large ganglion cells, with open radiate branching patterns, process fast, transient impulse trains and in all vertebrate retinas are concerned with motion detection and alerting the animal to threatening, moving visual imagery. While small bushy ganglion cell types are concerned with processing small stationary, fine detail in tonically activated messages in all species.
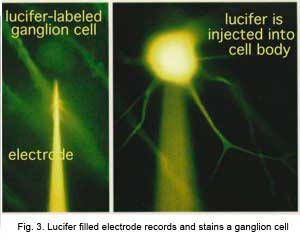
In the forties, Polyak (1941) produced a phenomenal description of the Golgi-impregnated neurons of the primate retina and therein he gave us a good classification of ganglion cell types (see chapter on Midget Pathways). So by the sixties we had a fairly extensive description and classification of the ganglion cells in mammalian and monkey retinas but all the data was based on vertical sections of stained ganglion cells (Cajal, 1892; Polyak, 1941; Brown and Major, 1966; Leicester and Stone, 1967; Boycott and Dowling, 1969; Shkolnik-Yarros, 1971). The advent of a technique to perform Golgi staining on wholemount retinas allowed a reinterpretation of many of the earlier classifications, because now we could see the entire dendritic tree of a ganglion cell. The wholemounts could also be compared with images of intracellularly injected cells after physiological recordings (Figs. 3 and 4 and see animation).
CLICK to see an animation of the iontophoresis of Lucifer dye into a ganglion cell via a microlectrode (Quicktime movie)
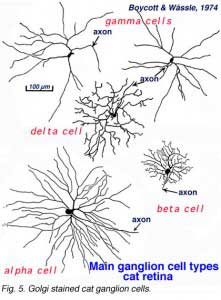
A particularly successful morphological classification scheme was proposed by Boycott and Wassle (1974). Three main classes of physiological response type were correlated with three morphological classes of ganglion cell in cat retina. Thus the alpha, beta, gamma and delta morphological ganglion cell types were considered to be the equivalents of the Y, X and W types of the physiology (Fig. 5, see below) (Boycott and Wassle, 1974; Enroth-Cugell and Robson, 1966; Cleland and Levick, 1974, Levick and Thibos, 1983, for a review). By comparison with Cajals drawings, it was clear that alpha and beta types were already described (see Cajal drawing of dog and ox retinal ganglion cells) and a further ten or more of Cajals (1892) types were lumped under the umbrella of gamma and delta groups.
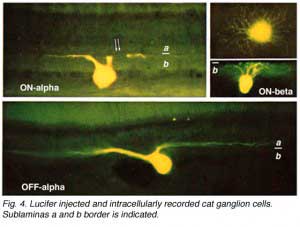
By 1978, we knew that alpha and beta cells of Boycott and Wassle (1974) could be subdivided into separate subtypes depending on whether they branch in sublamina a or sublamina b of the inner plexiform layer (Famiglietti and Kolb, 1976) (see chapter on inner plexiform layer). Sublamina a contained dendrites of cells with physiologically defined OFF-center receptive fields and sublamina b dendrites of cell with ON-center receptive fields (Nelson et al., 1978). A new classification of Golgi stained ganglion cells (Kolb et al., 1981) could now relate both alpha and beta cells to the functionally important sublamina scheme and further, described many new types of cell including and going beyond the gamma and delta cells. The 20 cell types over and above the alpha, beta and gamma cells were named from G4-G23 in order of cell body size and dendritic form (i.e. from smallest types, G4 to largest types at G23) (Kolb et al., 1981).
2. Alpha and beta ganglion cell types of mammalian retinas
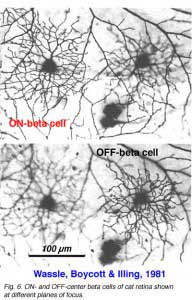
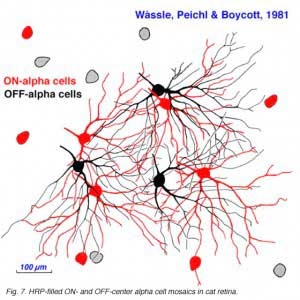
Alpha and beta ganglion cells of the cat retina are arranged in regular, superimposed bi-level mosaics across the whole retina (Wassle et al., 1981a,b). Both OFF-center (branching in sublamina a) and ON-center (branching in sublamina b) varieties of alpha and beta cells are arranged in a closely packed pattern with minimal overlap of their peripheral dendrites. ON- and OFF varieties are always paired as shown in Wassle and coauthors elegant papers (1984) (Fig. 6 and 7, below), and between an ON/OFF pair there is sampling of almost the same retinal space (Fig. 7).
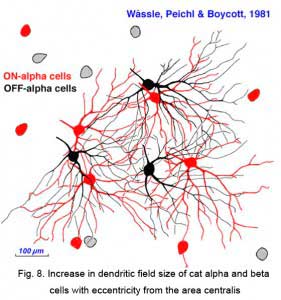
Both cell types have their smallest dendritic tree sizes at the area centralis of the cat retina (equivalent to the fovea of primate retinas) and radiate out from this central point with gradually expanding circular dendritic trees. To maintain the low overlap ratio of their dendritic trees, the cells with larger fields are consequently spaced at greater intervals from each other, i.e. density of the cells/mm2 of retina is inversely proportional to distance from the area centralis. This is shown in figure 8 (below) where the increase of ganglion cell size as shown with distance from the area centralis (a.c.): peripheral alpha and beta retinal ganglion cells are ten fold larger than their area centralis relatives.
3. Non alpha and non beta ganglion cell types in mammalian retinas
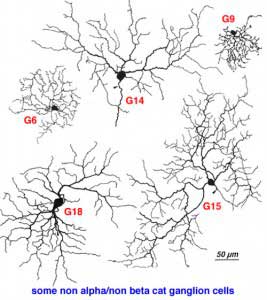
It has been estimated that between 50% and 60% of all ganglion cells in the cat retina are cells other than alpha and beta types (Fukuda and Stone, 1974; Stone and Fukuda, 1974). Alpha cells form 3% and beta cells 45-50 %. Kolb and coauthors (1981) Golgi study indicated that 21 different morphological types of ganglion cell were of the non-alpha/non beta cells classes (some of them are shown in Fig. 9, below, and others are shown in the human ganglion cell section). The G3 was the equivalent of the gamma cell of Boycott and Wassle (1974) (Fig. 5). G3 may be a concentrically organized type of W cell (Stone and Fukuda, 1974; Cleland and Levick, 1974b) and according to Kolb and coauthors (1981) comes in ON- and OFF-sublaminal branching subtypes. G19 cells equivalent to delta cells of Boycott and Wassle (1974) (Fig. 5) probably also come in the ON- and OFF subtypes (Wassle et al., 1987; Peichl, 1989; Wassle and Boycott, 1991). Diffuse wide-field G4 and monostratifying G18 cells are probably equivalent to two different OFF-tonic cells and G15 to an ON-directional cell (Fukuda et al., 1984). G19 which is similar to one type of delta cell of Boycott and Wassle (1974) may be an ON-tonic cell while G22 is almost certainly an ON/OFF phasic type (Fukuda et al., 1984). The newly described Q cell of Troy et al., (1995) may also be the physiological correlate of an ON-delta morphological type. Ganglion cells with the morphology of G21 and G23 are labelled by retrograde transport of tracers from brain visual centers: G21 from the accessory optic system (Farmer and Rodieck, 1982). The speculation is that G21 is a directionally selective ganglion cell because cells of the accessory optic system are reported to be directionally selective (Grasse and Cynader, 1980). A ganglion cell type that could be a G23 type has been labeled from retrograde markers injected into the retinal recipient zone (RRZ) in the pulvinar (Levinthal et al., 1980) and has been named the epsilon cell. However, the staining of epsilon cells has never been good enough to be sure which Golgi type it really is.
Other ganglion cell types that have been reported using tracer or other staining techniques than the Golgi method, include the medium-gamma cells, g1 and g2 cells (not clear which Golgi types these are) (Leventhal et al., 1985), Neuropeptide Y immunoreactive (NPY-IR) cells (Hutsler et al. 1993), ganglion cells of the geniculate wing (Pu et al., 1994), and indoleamine containing ganglion cells likened to delta cells (Wassle et al., 1987; Dacey, 1989).
4. Convergence of photoreceptors and second order neurons to cat retinal ganglion cells
The organization of the cat retina with a concentration of small-field beta cells in the area centralis allows the lowest convergence ratio of cone photoreceptors to ganglion cells. This is presumed to be the substrate for the high acuity pathways. The human (primate) retina culminates this trend with the development of the midget system and the photoreceptor to ganglion cell pathway to a one to one relationship of course (see chapter on midget pathways).
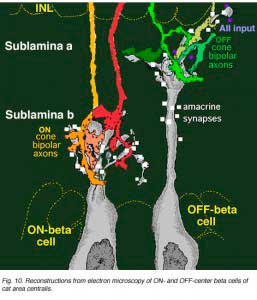
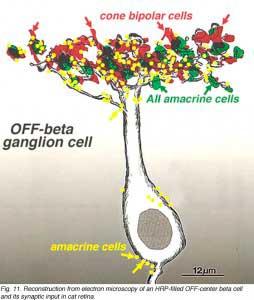
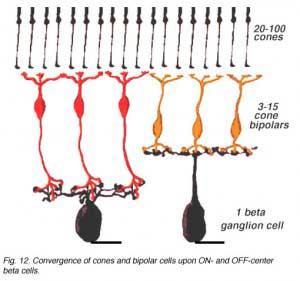
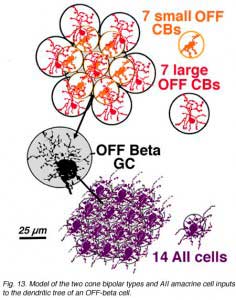
An electron microscope analysis of two area centralis beta cells in the cat, concluded that each ganglion cell received input from 3 or 4 cone bipolars which, in turn, received input from 4-8 cones (Fig. 10) (Kolb, 1979; Wassle et al., 1981). Another OFF-center beta ganglion cell has been calculated to have fourteen definite bipolar cell axon terminals inputting the dendrites (Fig. 11, four bipolar axons shown in red, arrows). Thus this type of ganglion cell would have convergence of approximately 100 cones through the 14 bipolar cells of two subtypes (see Figs. 12 and 13) (Fig. 13, small-field (yellow) and large-field (red) types of cone bipolar).
It is also calculated that about 14 AII amacrine cells (Fig. 13, purple cells), would also have input thereby converging 4200 rods through 280 rod bipolar cells to the 14 AII cells of input (Fig. 18). In addition, 28 to 41 other amacrine cells probably have synapses allowing even more convergence over a greater laterally spreading area than the ganglion cells dendritic tree diameter would have.
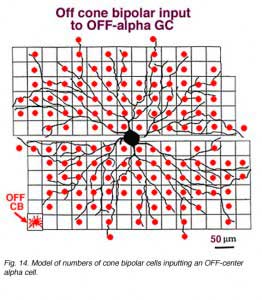
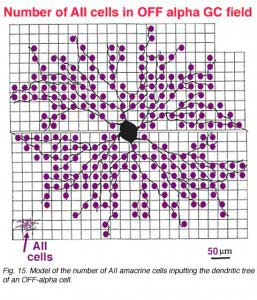
In the same vein, we have information on convergence of bipolar cells and amacrine cells upon both ON-center and OFF center alpha ganglion cells (Freed et al., 1990; Kolb and Nelson, 1993). In the model of the OFF-center alpha cell seen below (Figs. 14 and 15), cone bipolar synapses, from only one type of OFF-center cone bipolar (cb2 type, red dots), form 20% of the total synaptic input and the rest of the input is from various kinds of amacrine cells. A minimum of 142 bipolar cells, 256 AII amacrine cells and 1011 other amacrine cells, together, provide between 6000 and 10000 synapses on the dendritic tree of this OFF-center alpha ganglion cell. With a potential cone convergence of 5-15 cones per bipolar cell it is possible that 15000 cones could ultimately be involved in the capture area of this type of ganglion cell.
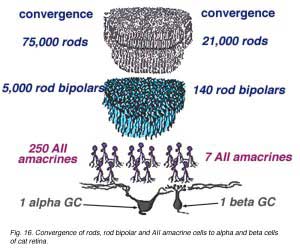
In the case of the rod system, the convergence of preceding neurons to the ganglion cell is enormous i.e calculated by Sterling et al (1988) to be 75,000 rods through 5000 rod bipolar cells to 250 AII amacrine cells to a single alpha cell (Fig. 11, below). The other amacrine cell input and their corresponding convergences is not capable of being assessed at the present time. Interestingly though, physiological measures of receptive field center sizes of alpha cells are not much larger than their dendritic tree sizes (Wassle et al., 1984; Nelson et al., 1993) (see below) despite enormous convergence and very wide lateral interactive potential.
5. Identity of amacrine cell types inputting cat alpha and beta ganglion cells
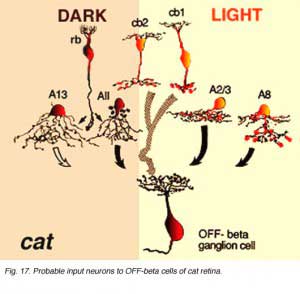
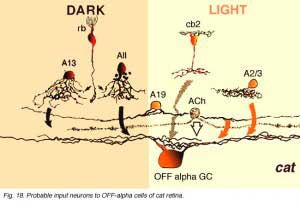
As shown in the summary diagrams (Figs. 17 and 18, below) recent evidence suggests that several different morphological types of amacrine cells, as well as bipolar cell types, have synaptic input to the OFF-beta and OFF-alpha ganglion cells.
AII amacrine cells are particularly evident to both OFF-center ganglion cell types (Figs. 17 and 18, AII) and as mentioned above (see Figs. 13 and 15, previous section). AII cells are known to be glycinergic (Pourcho and Goebel, 1985; Kallionatis and Marc, 1996). A small-field bistratified amacrine cell with morphological characteristics complementary to the AII known as A8 (Kolb, et al., 1981) (see chapter on roles of amacrine cells) has been studied for transmitter content (Pourcho and Goebel, 1985) and in intracellular recordings and marking (Kolb and Nelson, 1996). A8 is considered a glycinergic cell (Pourcho and Goebel, 1985). It synapses in sublamina a upon tufted dendrites and dendritic varicosities typical of the OFF-beta ganglion cell (Fig. 17, A8). However, these A8 amacrine profiles have not been seen to input large diameter OFF-alpha ganglion cell dendrites (Fig. 18). A13 cells are considered GABAergic cells (Pourcho and Goebel, 1986) and known to be postsynaptic to rod and cone bipolars and make synapses with the cell body and primary dendrites in sublamina aand b of both ganglion cell types as well (Kolb and Nelson, 1993; 1996) (Figs. 17 and 18, A13).
Small amacrine cells marked as A2/3 are shown on the summary diagrams (Figs. 17 and 18, A2/3). Pourcho and Goebel (1983; 1985) have clearly subdivided types A2, A3 and A4 by their neurotransmitter content. Thus A2 is probably gabaergic while A3 and A4 are apparently medium-intensity glycinergic. Since only A2 and A3 cell types branch in strata 1 and 2 while A4 branches deeper in stratum 3, it is more likely that the former two cells are the candidates for synaptic input to the OFF-alpha and OFF-beta cells. Freed and Sterling (1988) propose that glycinergic A4 amacrines are presynaptic to ON-center alpha cells: it is thus possible then that A3 are glycinergic counterpart amacrines reserved for the OFF-center variety of ganglion cell.
Some of the amacrine profiles synapsing upon the OFF-alpha ganglion cell have the morphology of A19 amacrine cells (Fig. 18, A19, arrows) (Kolb and Nelson, 1984; 1985; Freed et al., 1996). They are large-field with large-diameter dendrites tapering to fine, long processes covering 500 µm or more of field. Like most wide-field amacrine cells of the mammalian retin, A19 cells are thought to be GABAergic (Pourcho and Goebel, 1986) and ON-OFF in physiology (Freed et al., 1996). They run in lower sublamina a in S2 co-stratifying with OFF-center alpha cell dendrites. A19 cells have been characterized, by their content of neurotubules and their gap junctions to other A19s (Kolb and Nelson, 1984; 1985). Moreover, HRP-injected A19 cells have been seen to run along and across large diameter alpha cell dendrites in sublamina a making intermittent synapses (Freed et al., 1996).
Amongst the many wide-field amacrine synapses upon OFF center alpha cells, some are probably from acetylcholine containing or “starburst” amacrine cells (Famiglietti 1983; Tauchi and Masland, 1984; Vaney, 1990) (Fig. 18, ACh, stippled cell). Vardi and co-workers (1989) showed by lucifer filling alpha ganglion cells and immunocytochemically staining cholinergic amacrine cells, that the latter amacrine’s distal varicose dendrites run in bundles and loops along and across alpha cell dendrites in strata 2 and 3 of the IPL having ample opportunity for synaptic interaction. There is also convincing evidence for physiological effects of acetylcholine upon Y ganglion cells (Ikeda, 1985; Schmidt et al., 1987; Straschill and Perwein, 1973) and evidence that alpha cell dendrites contain ACh receptor sites (Kaneda et al., 1995). Thus it seems probable that the two cholinergic amacrine cell types contributing to a and b sublaminae in cat retina are providing synaptic inputs to OFF- and ON-center alpha ganglion cells respectively.
6. Primate ganglion cells
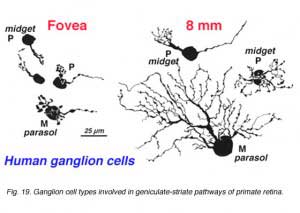
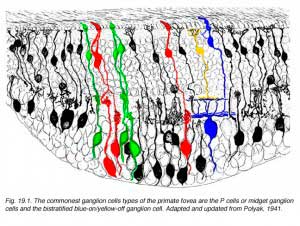
There are at least 18 different morphological types of ganglion cell in the human retina. All but three of these correspond to cells in the cat retina and have, where possible, been given the same G number. Some of the cat types have not been seen in human retina but undoubtedly they are there. Special names are reserved for P and M ganglion cells that are specific to the human high visual acuity and color processing systems. They are thought to be specialized evolutionary correlates of beta and alpha cells respectively (Leventhal et al., 1981) and comprise the major input to the geniculo-striate areas (Perry, 1981; 1984a, 1984b; Shapley, 1986). Thus P cells are considered to be mostly midget ganglion cells and equivalent to beta cells of cat, and project to the Parvocellular layers of the LGN. M cells, on the other hand, probably equivalent to alpha cells of cat and project to the Magnocellular layers of the LGN (Fig. 19, below).
CLICK to see a focus series of two Golgi stained P cells in human retina (Quicktime movie)
Polyak (1941) first described the midget ganglion cells and considered them to form the majority of the ganglion cell types of monkey central retina. More recent retrograde staining studies indicate that ganglion cells with medium-size cell bodies and the smallest dendritic trees, putting them into a “midget” category of cell, comprise 80% of the ganglion cell population in the monkey retina, and these cells project to the parvocellular layers of the LGN (Perry, 1984a). The question then arises as to how many cell types correspond to these parvocellular projecting ganglion cells. Polyak (1991) describes midget ganglion cells quite clearly as the ganglion cells with the smallest dendritic trees never much exceeding the diameter of their cell body which is 9-12 µm. It is clear that the cells of the fovea meet this criterion (Figure 19). However outside of the fovea and beyond 3mm of eccentricity the midget ganglion cells can have dendritic tree sizes as large as 100 µm (Rodieck et al, 1985; Watanabe and Rodieck, 1989; Dacey, 1993).
Physiologically we suspect by virtue of their being single-cone-connected via their exclusive connection to single midget bipolar cells in the foveal area (Kolb and DeKorver, 1991; Calkins et al., 1994) that P cells are the opponent chromatic units, or Type 1 cells of Weisel and Hubel, of the LGN (Weisel and Hubel, 1966; Gouras, 1968). Outside of the fovea where they clearly are receiving input from more than one midget bipolar cell by virtue of their increased dendritic tree size midget ganglion cells or P cells may become broader band (Gouras and Zrenner, 1991). We suspect that the P cells always receive input through midget bipolar cells but probably from more than one midget bipolar cell. In peripheral retina the P cell dendritic trees become large enough for axons of 3-5 midget bipolar cells to to have input. It remains to be seen whether chromatic specificity can remain under these conditions.
There is general agreement that the magnocellular-projecting ganglion cells are the parasol cells that Polyak described (1941). Although some of Polyak’s giant cells are by cell body size and dendritic tree size characteristics undoubtedly the equivalent of Golgi-stained M cells in far peripheral retina (Kolb et al., 1992; Rodieck et al., 1985; Watanabe and Rodieck., 1989) and of the large-bodied, multibranched reduced silver stained cells of Silveira and Perry (1991).
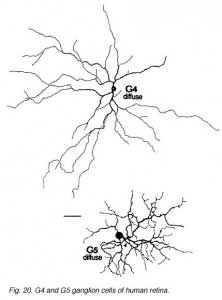
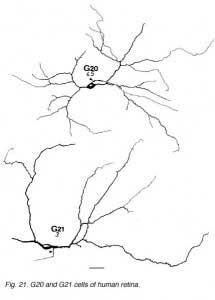
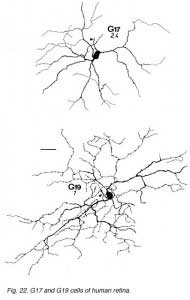
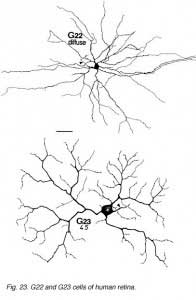
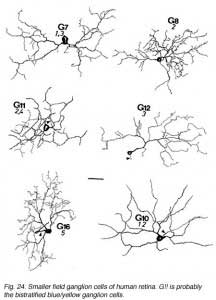
The other ganglion cells in human that are not in the geniculo-striate projections correspond to some of those retrogradely-filled in Perry and co-workers studies of cells projecting to the midbrain (Perry, 1984) (Figs. 20-23). Two of these varieties in macaque retina may be G22 and G23 cells (Fig. 23). The G19 is equivalent to the cell in cat retina that has been stained by intracellular dye-injection and is apparently monoamine accumulating (Fig. 22) (Dacey, 1989). Ganglion cells with complex receptive field characteristics such as directional selectivity (DS) may be present in human retina too. Thus G11 (Fig. 24) is a bistratified cell that could correspond to the rabbit ON-OFF DS cell and G22 (Fig. 23) to an ON directionally selective cell (Amthor et al., 1989b). Ganglion cells selective for orientation and uniformity could correspond to G8 (Fig. 24) and G17 (Fig. 22) respectively, in human retina (Amthor et al., 1989b). Several of the concentric sluggish cells of the rabbit are also seen in human Golgi-stained counterparts, for example G3 and G20 could be an ON-center varieties and G7 and G4 OFF-center varieties (Figs. 21-24) (Amthor et al., 1989a).
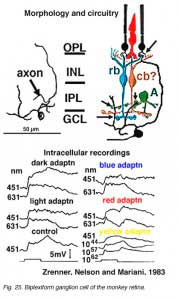
Only two physiologically recorded and stained monkey ganglion cells have been reported so far. One is the little understood biplexiform ganglion cell (Mariani, 1982; Zrenner et al., 1983) (Fig. 25) which gives a sustained ON-center physiology, representative of its direct input from rod photoreceptors. We will return to this fascinating ganglion cell type later (see below). The other ganglion cell that has been recorded from in monkey is the blue/yellow opponent ganglion cell projecting to the parvocellular layers of LGN (Dacey and Lee, 1994) (see chapter on S-cone pathways).
7. Rabbit ganglion cells
The rabbit retina has been extensively studied because it is a more accessible retina in respects to isolation, staining and recordings than some other mammalian retinas. The retina can be isolated and kept alive for hours of experimental study with simple perfusion techniques. Rabbit retina was originally studied for the property of directional selectivity in ganglion cells. Because this retina has a visual streak specialization, it was found quite early on (Barlow and Levick, 1965) that many of the the ganglion cells were sensitive to motion and direction of the movement of visual stimuli.
Interestingly there has never been a systematic classification of the different types of rabbit ganglion cell based on a morphological technique like the Golgi. However, the intracellular staining and physiological approaches have yielded a very nice functional classification scheme that supercedes the need for a Golgi study nowadays.
Ganglion cells equivalent to ON and OFF-alpha types in cat are also seen in rabbit (Peichl et al., 1987; Amthor et al., 1989a). They have both a similar physiology and dendritic field size and shape although in rabbit their cell bodies may not be quite as large in size as in cat retina. equivalent beta cell types in rabbit are a little more equivocal. Cells that respond with the linear, brisk sustained concentric receptive field organization that come in ON and OFF-center pairs, are seen in rabbit and their appearance is rather similar to peripheral beta cells of cat. They have, however, smaller cell body sizes, again like the alpha cell comparison (Amthor et al., 1989a). Because no cell with the exact same morphology as a central beta cell, even on the visual streak of rabbit retina, has been seen (Amthor et al., 1989a) some suggest that there is no real equivalent of the beta cell in rabbit (Wassle and Boycott, 1991). The question remains an open one, because the rabbit visual system has a different organization than the cats, with a horizontal streak in the retina, no binocular overlap of the visual fields and projections to visual centers in the tectum rather than the geniculo-striate.
Other ganglion cells of rabbit, with concentric receptive field physiology but more sluggish response patterns, are also demonstrated in Amthor and co-authors work (1989a). Many of these types are similar to the non-alpha/non-beta cell types of the cat in morphology. However, the correlation between the ganglion cell physiology of the two species is difficult to make at the present time. Some comparisons between the ganglion cells of the human retina and those of the rabbit have been attempted (Kolb et al., 1992). Interestingly, a great many more similarities exist between ganglion cell types of the ground squirrel and rabbit retinas (Linberg et al., 1996).
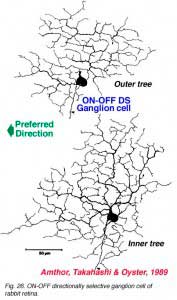
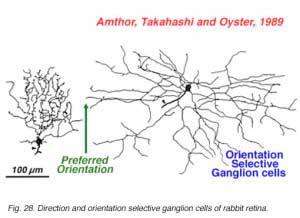
The more unusual feature detectors of the rabbit retina are the motion sensitive, orientation and direction sensitive ganglion cells (Amthor et al., 1989b). The bistratified ON-OFF directional selective (DS) ganglion cells of the rabbit retina have attracted a lot of attention mainly because many aspects of the circuitry have been elucidated (Fig. 26). For example the acetylcholine containing amacrine cells, also called starburst cells, are the major input cells (Masland, 1984; 1988; Famiglietti, 1987; 1991) (see chapter on roles of amacrine cells). The effects of antagonists of acetylcholine have been known for a long time to eliminate directional selectivity in the rabbit retina, and amacrines containing GABA are also known to be involved (Wyatt and Daw, 1976; Daw and Ariel, 1981). The fascinating aspect of the ON-OFF directionally selective ganglion cells of rabbit is that they are bistratified and have the two layers of their dendrites oriented orthogonal to each other (see Fig. 26). The orientation of the outer dendritic tree (i.e. the OFF layer of dendrites) is favored to be with the preferred direction of the cells response to a moving target (Amthor et al., 1989b).
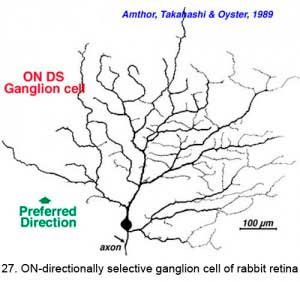
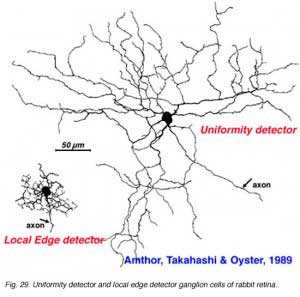
The other type of directionally selective cell in rabbit is the ON-center type and its single layered dendritic tree in sublamina b (ON layer of the IPL) is not as obviously oriented with the preferred direction (Fig. 27). Other ganglion cell types that are prominent and well characterized in Amthor and coworkers studies (1989b) in the rabbit retina, include orientation selective, local edge detectors and uniformity detectors (suppressed by contrast) (Figs. 28 and 29). It is particularly interesting that each of the different types of ganglion cells have a distinctly different morphology, primarily concerning the substructure of their dendrites. Many of them have another feature in common in that they typically show an orientation of the whole dendritic tree away from the cell body in an asymmetric pattern (see Figs. 26-29). All the details of these morphologies can be found in the elegant papers of Amthor, Takahashi and Oyster (1989a,b) and the reader is encouraged to look there.
8. Mouse ganglion cells
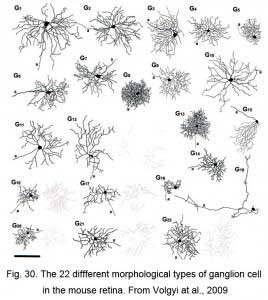
Recently several studies have been directed at describing the different morphological types of ganglion cell in the mouse retina (Sun et al., 2002; Badea and Nathans, 2004; Kong et al., 2005; Coombs et al., 2006 and Volgyi et al., 2009). These studies have been done by intracellular iontophoresis of neurobiotin or Lucifer labeling of GFP labeled retina or genetic labeling with, as can be imagined, varying numbers of ganglion cell types found, probably due to differences in experimental techniques. Some studies say there are 11-12 clusters of ganglion cell types but others find 17-22 different cell types. The Volgyi study seems the most complete (Volgyi et al., 2009) where 22 different ganglion cells types, reminiscent of the cat and human retina’s ganglion cells (Kolb et al., 1981; Kolb et al., 1992), are described. The Volgyi study is also most complete in describing the coupling between homologous ganglion cell types and heterologous coupling between ganglion cells and amacrine cells.
Thus figure 30 indicates the 22 different types of ganglion cell seen in the mouse retina. Cells are classified on the usual morphometric parameters such as cell body size, field size, dendritic morphology and stratification level. As can be seen the ganglion cells vary from very large bodied and large dendritic fields to small bodies and small dendritic fields. Several types are bistratified (i.e. G12, G16, G17 and G20-22) some of which may be directionally slective cells. One type (G15) has an asymmetric dendritic tree and clearly corresponds to Meister’s mouse ganglion cells that respond to upward image motion in the direction of the dendrites (Kim et al., 2008). Of these 22 ganglion cell types, 16 types were either coupled to other ganglion cell neighbors (of the same type), or coupled to large field amacrine cell types.
Vaney showed that many types of cells including amacrine and ganglion cells were tracer coupled in the vertebrate retina (Vaney, 1991). In the Bloomfield study here referred to (Fig. 30), the full extent of tracer coupling (thought to indicate electrical coupling) has been revealed with often good enough spread of tracer to show the morphologies of the coupled elements. Thus most of the ganglion cells are coupled to wide-field amacrines, some of which are the polyaxonal types. Ganglion cells that have dendritic trees in sublamina b are coupled to displaced wide-field amacrine cells and those in sublamina a are coupled to normally placed amacrine cells of the INL. Six ganglion cell types showed no coupling to either homologous ganglion cells or amacrine cells (G5, G9, G12, G14, G19, G22). It is clear that coupling between ganglion cell types is responsible for synchronized firing patterns. The populations of amacrine cells may also link each other to particular ganglion cell types in order to increased correlated activity both spatially and temporally. However, as dendritic field and receptive field sizes of ganglion cells are about the same, it seems that the function of coupling of amacrines to ganglion cells does not act, as does coupling between horizontal cells for example, to increase receptive field size.
9. References
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol. 1989;280:97–121. [PubMed]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol. 1989;280:72–96. [PubMed]
- Badea, T.C. and Nathans, J. (2004) Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol., 480: 331-351. [PubMed]
- Barlow HB, Levick WR. The mechanism of directionally selective units in the rabbit’s retina. J Physiol. 1965;178:477–504. [PubMed]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philos Trans. R. Soc. B. 1969;255:109–184.
- Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol. 1974;240:397–419. [PubMed] [Free Full text in PMC]
- Brown JE, Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966;15:70–78. [PubMed]
- Cajal SR. . In: Thorpe SA, Glickstein M, translators. 1892. The structure of the retina. Springfield (IL): Thomas. 1972
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses.Nature. 1994;371:70–72. [PubMed]
- Cleland BG, Levick WR. Brisk and sluggish concentrically organised ganglion cells in the cat’s retina. J Physiol. 1974;240:421–456. [PubMed] [Free Full text in PMC]
- Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat’s retina. J Physiol. 1974;240:457–492. [PubMed] [Free Full text in PMC]
- Dacey DM. Monoamine-accumulating ganglion cell type of the cat’s retina. J Comp Neurol. 1989;288:59–80. [PubMed]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355. [PubMed]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathways in primate retina originates from a distinct bistratified ganglion cell. Nature. 1994;367:731–735. [PubMed]
- Daw NW, Ariel M. Effect of synaptic transmitter drugs on receptive fields of rabbit ganglion cells. Vision Res. 1981;21:1643–1648. [PubMed]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966;187:517–552. [PubMed] [Free Full text in PMC]
- Famiglietti EV, Kolb H. Structural basis of “On-” and “Off-” centre responses in retinal ganglion cells. Science. 1976;194:193–195. [PubMed]
- Famiglietti EV. ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells of rabbit retina. Brain Res. 1983;261:138–144.[PubMed]
- Famiglietti EV. Starburst amacrine cells in cat retina are associated with bistratified, presumed directionally selective, ganglion cells. Brain Res. 1987;413:404–408.[PubMed]
- Famiglietti EV. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991;309:40–70. [PubMed]
- Farmer SG, Rodieck RW. Ganglion cells of the cat accessory optic system: morphology and retinal topography. J Comp Neurol. 1982;205:190–198. [PubMed]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988;8:2303–2320. [PubMed]
- Freed MA, Pflug R, Kolb H, Nelson R. ON-OFF amacrine cells in cat retina. J Comp Neurol. 1996;364:556–566. [PubMed]
- Fukuda Y, Stone J. Retinal distribution and central projections of Y-, X- and W-cells of the cat’s retina. J Neurophysiol. 1974;37:749–772. [PubMed]
- Fukuda Y, Hsiao C-F, Watanabe M, Ito H. Morphological correlates of physiologically identified Y-, X-, and W-cells in cat retina. J Neurophysiol. 1984;52:999–1013. [PubMed]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968;199:533–547. [PubMed] [Free Full text in PMC]
- Gouras P, Zrenner E. Color coding in primate retina. Vision Res. 1981;21:1591–1598. [PubMed]
- Grasse KL, Cynader MS. Electrophysiology of medial terminal nucleus of accessory optic system in the cat. J Neurophysiol. 1982;48:490–504. [PubMed]
- Hutsler JJ, White CA, Chalupa LM. Neuropeptide Y immunoreactivity identifies a group of gamma-type retinal ganglion cells in the cat. J Comp Neurol.1993;336:468–480. [PubMed]
- Ikeda H. Transmitter action at cat retinal ganglion cells. Prog. Ret. Res. 1985;4:1–32.
- Kalloniatis M, Marc RE, Murry RF. Amino acid signatures in the primate retina. J Neurosci. 1996;16:6807–6829. [PubMed]
- Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina. Jpn J Physiol. 1995;45:491–508. [PubMed]
- Kim, I.J., Zhang, Y., Yamagata, M., Meister, M. and Sanes, J.R. (2008) Molecular identification of a retinal cell type that responds to upward motion. Nature 4452: 478-482. [PubMed]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. [PubMed]
- Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21:1081–1114. [PubMed]
- Kolb H, Nelson R. Neural architecture of the cat retina. Prog. Ret. Res. 1984;3:21–60.
- Kolb H, Nelson R. (1985) Functional neurocircuitry of amacrine cells in the cat retina. In: Gallego A, Gouras P, editors. Neurocircuitry of the retina: a Cajal memorial. New York: Elsevier Press. pp. 215-232.
- Kolb H, DeKorver L. Midget ganglion cells of the parafovea of the human retina: A study by electron microscopy and serial section reconstructions. J Comp Neurol.1991;303:617–636. [PubMed]
- Kolb H, Linberg KA, Fisher SK. The neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318:147–187. [PubMed]
- Kolb H, Nelson R. Off-alpha and off-beta ganglion cells in the cat retina. II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol.1993;329:85–110. [PubMed]
- Kolb H, Nelson R. Hyperpolarizing, small-field, amacrine cells in cone pathways of cat retina. J Comp Neurol. 1996;371:415–436. [PubMed]
- Kong, J-H., Fish, D.R., Rockhill, R.L. and Masland, R.H. (2005) Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J. Comp. Neurol. 489: 293-310. [PubMed]
- Leicester J, Stone J. Ganglion, amacrine and horizontal cells of the cat’s retina. Vision Res. 1967;7:695–705. [PubMed]
- Leventhal AG, Keens J, Tork I. The afferent ganglion cells and cortical projections of the Retinal Recipient Zone (RRZ) of the cat’s ‘Pulvinar Complex’. J Comp Neurol. 1980;194:535–554. [PubMed]
- Leventhal AG, Rodieck RW, Dreher B. Central projections of the cat retinal ganglion cells. J Comp Neurol. 1985;237:216–226. [PubMed]
- Leventhal AG, Rodieck RW, Dreher B. Retinal ganglion cell classes in the old world monkey: morphology and central projections. Science. 1981;213:1139–1142.[PubMed]
- Levick WR, Thibos LN. Receptive fields of cat ganglion cells: classification and construction. Prog Retinal Res. 1983;2:267–320.
- Linberg KA, Suemune S, Fisher SK. Retinal neurons of the California ground squirrel, Sperophilus beecheyi: A Golgi study. J Comp Neurol. 1996;365:173–216.[PubMed]
- Mariani AP. Biplexiform cells: ganglion cells of the primate retina that contact photoreceptors. Science. 1982;216:1134–1136. [PubMed]
- Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:121–139. [PubMed]
- Masland RH. Amacrine cells. Trends Neurosci. 1988;11:405–410. [PubMed]
- Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for On-and Off-centre ganglion cells in cat retina. J Neurophysiol.1978;41:472–483. [PubMed]
- Nelson R, Kolb H, Freed M. Off-alpha and Off-beta ganglion cells in cat retina. I. Intracellular electrophysiology and HRP stains. J Comp Neurol. 1993;329:68–84.[PubMed]
- Peichl L, Buhl EH, Boycott BB. Alpha ganglion cells in the rabbit retina. J Comp Neurol. 1987;263:25–41. [PubMed]
- Perry VH, Cowey A. The morphological correlates of X- and Y-like retinal ganglion cells in the retina of monkeys. Exp Brain Res. 1981;43:226–228. [PubMed]
- Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. [PubMed]
- Perry VH, Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984;12:1125–1137.[PubMed]
- Polyak SL. The retina. Chicago: University of Chicago Press. 1941
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist (3H)muscimol: a combined Golgi and autoradiographic study.J Comp Neurol. 1983;219:25–35. [PubMed]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of 3(H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol.1985;233:473–480. [PubMed]
- Pu M, Berson DM, Pan T. Structure and function of the retinal ganglion cells innervating the cat’s geniculate wing: An in vitro study. J Neurosci. 1994;14:4338–4358. [PubMed]
- Rodieck RW, Binmoeller KF, Dineen JT. Parasol and midget ganglion cells of the human retina. J Comp Neurol. 1985;233:115–132. [PubMed]
- Schmidt M, Humphrey MF, Wassle H. Action and localization of acetylcholine in the cat’s retina. J Neurophysiol. 1987;58:997–1015. [PubMed]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. Trends Neurosci. 1986;9:229–235.
- Shkolnik-Yarros EG. Neurons of the cat retina. Vision Res. 1971;11:7–26. [PubMed]
- Silveira LCL, Perry VH. The topography of magnocellular projecting ganglion cells (M-ganglion cells) in the primate retina. Neuroscience. 1991;40:217–237.[PubMed]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–642. [PubMed]
- Stone J, Fukuda Y. Properties of cat retinal ganglion cells: Comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974;37:722–748. [PubMed]
- Straschill M, Perwein J. The inhibition of retinal ganglion cells by catecholamines and g-aminobutyric acid. Pflugers Arch. 1969;312:45–54. [PubMed]
- Sun, W,, Li, X., He, S (2002) Large-scale morphological survey of mouse retinal ganglion cells. J.Comp. Neurol. 451: 115-126. [PubMed]
- Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:101–119. [PubMed]
- Troy JB, Schweitzer-Tong DE, Enroth-Cugell Ch. Receptive-field properties of Q retinal ganglion cells of the cat. Vis Neurosci. 1995;12:285–300. [PubMed]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog. Ret. Res. 1990;9:49–100.
- Vaney, D.I. (1991) Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci. Lett. 125: 187-190. [PubMed]
- Vardi N, Masarachia PJ, Sterling P. Structure of the starburst amacrine network in the cat retina and its association with alpha ganglion cells. J Comp Neurol.1989;288:601–611. [PubMed]
- Volgyi, B., Chheda, S. and Bloomfield, S.A. (2009) Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J. Cop. Neurol. 512: 664-687. [PubMed]
- Wassle H, Peichl L, Boycott BB. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981;212:157–175.[PubMed]
- Wassle H, Boycott BB, Illing R-B. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci. 1981;212:177–195. [PubMed]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. [PubMed]
- Wassle H, Voigt T, Patel B. Morphological and immunocytochemical identification of indoleamine-accumulating neurons in the cat retina. J Neurosci. 1987;7:1574–1585. [PubMed]
- Watanabe M, Rodieck RW. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989;289:434–454. [PubMed]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966;29:1115–1156. [PubMed]
- Wyatt HJ, Daw NW. Specific effects of neurotransmitter anatagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. [PubMed]
- Zrenner E, Nelson R, Mariani A. Intracellular recordings from a biplexiform ganglion cell in macaque retina, stained with horseradish peroxidase. Brain Res.1983;262:181–185. [PubMed]